Abstract
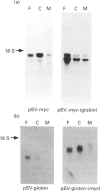
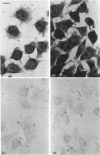
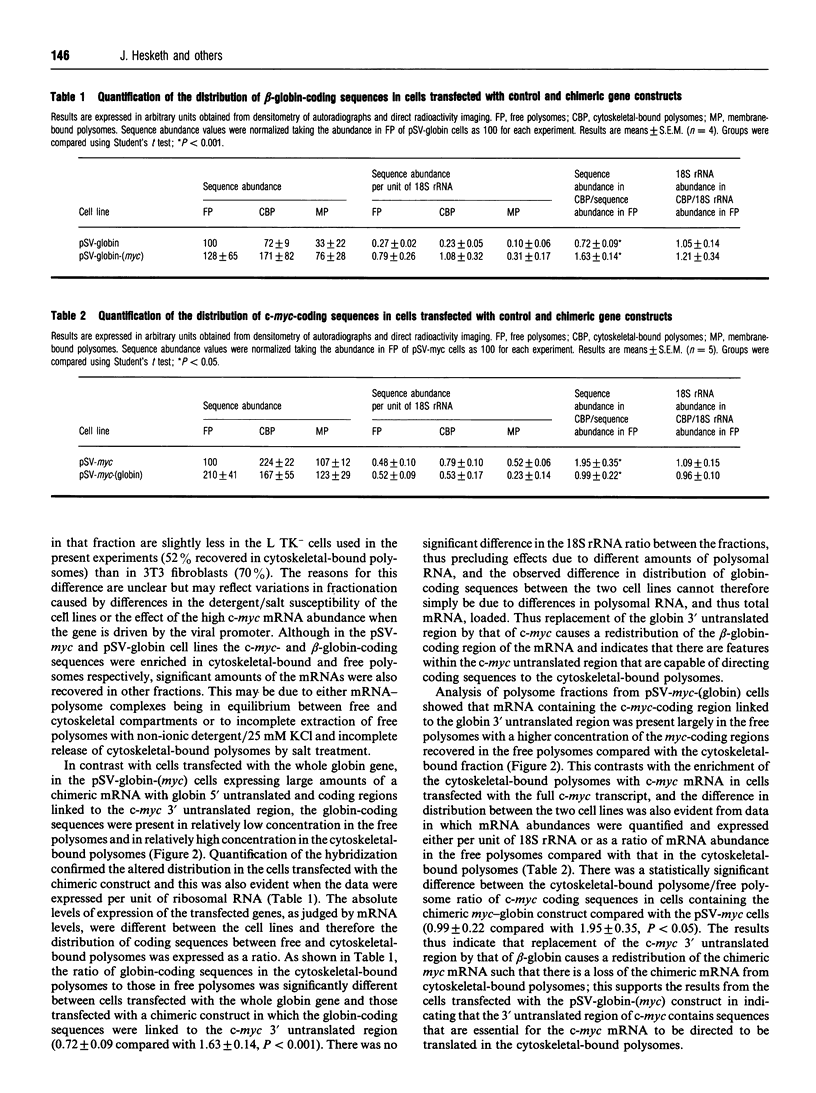
The influence of the 3' untranslated region on mRNA localization was investigated by measuring the distribution of myc, beta-globin and hybrid myc-globin mRNAs between free, cytoskeletal-bound and membrane-bound polysomes in cells transfected with either control or chimeric gene constructs. c-myc sequences and beta-globin-coding sequences linked to the myc 3' untranslated region were present at greatest enrichment in cytoskeletal-bound polysomes. beta-Globin mRNA and myc-coding sequences linked to the beta-globin 3' untranslated region were recovered largely in the free polysomes. In situ hybridization confirmed that replacement of the c-myc 3' untranslated region by that of globin caused a relocalization of the mRNA. The results suggest that mRNA localization in differentiated eukaryotic cells depends on a mechanism that involves directional information in the 3' untranslated region of mRNAs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird R. C., Sells B. H. Cytoskeleton involvement in the distribution of mRNP complexes and small cytoplasmic RNAs. Biochim Biophys Acta. 1986 Dec 18;868(4):215–225. doi: 10.1016/0167-4781(86)90057-6. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnieu A., Piechaczyk M., Marty L., Cuny M., Blanchard J. M., Fort P., Jeanteur P. Sequence determinants of c-myc mRNA turn-over: influence of 3' and 5' non-coding regions. Oncogene Res. 1988 Sep;3(2):155–166. [PubMed] [Google Scholar]
- Bonnieu A., Roux P., Marty L., Jeanteur P., Piechaczyk M. AUUUA motifs are dispensable for rapid degradation of the mouse c-myc RNA. Oncogene. 1990 Oct;5(10):1585–1588. [PubMed] [Google Scholar]
- Campbell D. A., du Bois R. M., Butcher R. G., Poulter L. W. The density of HLA-DR antigen expression on alveolar macrophages is increased in pulmonary sarcoidosis. Clin Exp Immunol. 1986 Jul;65(1):165–171. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cole M. D., Mango S. E. cis-acting determinants of c-myc mRNA stability. Enzyme. 1990;44(1-4):167–180. doi: 10.1159/000468755. [DOI] [PubMed] [Google Scholar]
- Davis I., Ish-Horowicz D. Apical localization of pair-rule transcripts requires 3' sequences and limits protein diffusion in the Drosophila blastoderm embryo. Cell. 1991 Nov 29;67(5):927–940. doi: 10.1016/0092-8674(91)90366-7. [DOI] [PubMed] [Google Scholar]
- Erickson J. M., Rushford C. L., Dorney D. J., Wilson G. N., Schmickel R. D. Structure and variation of human ribosomal DNA: molecular analysis of cloned fragments. Gene. 1981 Dec;16(1-3):1–9. doi: 10.1016/0378-1119(81)90055-x. [DOI] [PubMed] [Google Scholar]
- Farquharson C., Milne J., Loveridge N. Mitogenic action of insulin-like growth factor-I on human osteosarcoma MG-63 cells and rat osteoblasts maintained in situ: the role of glucose-6-phosphate dehydrogenase. Bone Miner. 1993 Aug;22(2):105–115. doi: 10.1016/s0169-6009(08)80222-x. [DOI] [PubMed] [Google Scholar]
- Hesketh J. E., Campbell G. P., Whitelaw P. F. c-myc mRNA in cytoskeletal-bound polysomes in fibroblasts. Biochem J. 1991 Mar 1;274(Pt 2):607–609. doi: 10.1042/bj2740607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh J. E., Pryme I. F. Interaction between mRNA, ribosomes and the cytoskeleton. Biochem J. 1991 Jul 1;277(Pt 1):1–10. doi: 10.1042/bj2770001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell. 1986 May 9;45(3):407–415. doi: 10.1016/0092-8674(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Struhl G. cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature. 1988 Dec 8;336(6199):595–598. doi: 10.1038/336595a0. [DOI] [PubMed] [Google Scholar]
- Mowry K. L., Melton D. A. Vegetal messenger RNA localization directed by a 340-nt RNA sequence element in Xenopus oocytes. Science. 1992 Feb 21;255(5047):991–994. doi: 10.1126/science.1546297. [DOI] [PubMed] [Google Scholar]
- Pondel M. D., King M. L. Localized maternal mRNA related to transforming growth factor beta mRNA is concentrated in a cytokeratin-enriched fraction from Xenopus oocytes. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7612–7616. doi: 10.1073/pnas.85.20.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell B., Dix D. J. Mechanisms for intracellular distribution of mRNA: in situ hybridization studies in muscle. Am J Physiol. 1992 Jan;262(1 Pt 1):C1–C8. doi: 10.1152/ajpcell.1992.262.1.C1. [DOI] [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Sundell C. L., Singer R. H. Actin mRNA localizes in the absence of protein synthesis. J Cell Biol. 1990 Dec;111(6 Pt 1):2397–2403. doi: 10.1083/jcb.111.6.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedeler A., Pryme I. F., Hesketh J. E. The characterization of free, cytoskeletal and membrane-bound polysomes in Krebs II ascites and 3T3 cells. Mol Cell Biochem. 1991 Feb 2;100(2):183–193. doi: 10.1007/BF00234167. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987 Dec 4;51(5):861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]
- Yisraeli J. K., Melton D. A. The material mRNA Vg1 is correctly localized following injection into Xenopus oocytes. Nature. 1988 Dec 8;336(6199):592–595. doi: 10.1038/336592a0. [DOI] [PubMed] [Google Scholar]