Table 3.
Main metabolic pathways and related enzymes of natural aporphine alkaloids.
| Natural Compound | Structure | Phase I reaction | Phase II reaction | Ref. |
|---|---|---|---|---|
| Apomorphine | 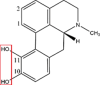 |
Oxidation: OH→=O | Glucuronidation: 10- and 11-O-monoglucuronides O-methylation: OH → OCH3 (COMT) |
[76] |
| Nuciferine | 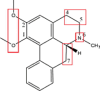 |
N-oxidation: (CYP 3A4); Demethylation: OCH3 → OH (CYP 1A2, 2A6, and 2C8)Dehydrogenation: C4-C5 and C6a-C7 (CYP 3A4) Hydroxylation: C5 → –OH (CYP 3A4) |
N-glucuronidation (UGT1A4) | [65], [144] |
| Magnoflorine | 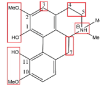 |
O-demethylation: 2,10-OMe N-demethylation Dehydrogenation: C4-C5 and C6a-C7, O-ketonization: 4-OHHydroxylation: C-3, C-4, C-9 |
O-glucosylation, O-glucuronidationO-sulfation |
[68] |
| Dicentrine | 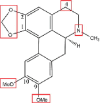 |
N-oxidationN or O or O, O-demethylation (1-OCH2O-2)Hydroxylation: C-3, C-4 |
Glucuronidation Glucosylation O-methylation: OH → OMe (C-4) |
[82] |
| Boldine | 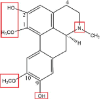 |
N-demethylation (N-demethyl-boldine-O-sulphate) | Glucuronidation and Sulphation(boldine-O-glucuronide, boldine-O-sulphate and disulphate, boldine-O-glucuronide-O-sulphate) | [83] |
| Isoboldine | 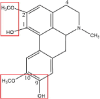 |
– | Glucuronidation Sulfonation: OH → SO3H |
[85] |
| Norisoboldine | 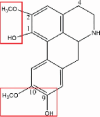 |
– | Glucuronidation: OH → GluA, Sulfonation: OH → SO3H (noriosboldine-1-O-β-d-glucuronide, norisoboldine-9O-α-d-glucuronide and disulfuric acid-1, 9-norisoboldine ester) |
[84] |
| Thalicarpine | 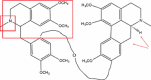 |
N-demethylation Aporphine ring oxidation Benzylic oxidationbenzylic reduction |
– | [87] |
COMT: catechol-O-methyl transferase; CYP: Cytochrome P450 enzymes; UGT: UDP-glucuronosyltransferases.
