Abstract
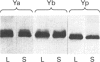
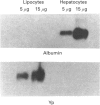
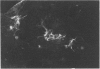
Hepatocytes in vivo express Alpha and Mu but not Pi forms of glutathione S-transferase (GST). GST P (a fetal Pi form) appears in rat hepatocytes after 2 days in primary culture, which suggests that hepatocytes may undergo dedifferentiation [Abramovitz, Ishigaki and Listowsky (1989) Hepatology 9, 235-239]. However, in this and other studies, primary rat hepatocyte cultures were shown by immunohistochemistry to contain significant numbers of lipocytes (Ito cells). Freshly isolated lipocytes contained GST activity when assayed with chlorodinitrobenzene (680 nmol/min per mg), and expression of Alpha, Mu and Pi forms of GST was detected by Western-blot analysis. Expression of GST P persisted during culture of the lipocytes. In situ hybridization of the cultured cells was performed to define whether hepatocytes, lipocytes or both expressed the enzyme. Lipocytes in culture contained abundant GST P transcripts. Hepatocytes contained no GST P transcripts after 12 h in culture, and after 24 h, only a few hepatocytes expressed this enzyme. After 48 h in culture all hepatocytes contained GST P transcripts, and the number of transcripts continued to increase up until 72 h. Therefore, in freshly isolated preparations of hepatocytes and early in hepatocyte culture, measurable levels of GST P protein or message appeared to reflect the presence of lipocytes. After 48 h in culture almost all of the GST P reflected expression by the hepatocytes. Lipocytes constitutively expressed Alpha-, Mu- and Pi-class GSTs and had significant intracellular levels of GSH (5.2 nmol/mg of protein). Lipocytes are capable therefore of detoxifying a number of injurious compounds.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitz M., Ishigaki S., Listowsky I. Differential regulation of glutathione S-transferases in cultured hepatocytes. Hepatology. 1989 Feb;9(2):235–239. doi: 10.1002/hep.1840090212. [DOI] [PubMed] [Google Scholar]
- Boyer T. D., Olsen E. Role of glutathione S-transferases in heme transport. Biochem Pharmacol. 1991 Jun 21;42(1):188–190. doi: 10.1016/0006-2952(91)90699-6. [DOI] [PubMed] [Google Scholar]
- Boyer T. D. The glutathione S-transferases: an update. Hepatology. 1989 Mar;9(3):486–496. doi: 10.1002/hep.1840090324. [DOI] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Diegelmann R. F., Guzelian P. S., Gay R., Gay S. Collagen formation by the hepatocyte in primary monolayer culture and in vivo. Science. 1983 Mar 18;219(4590):1343–1345. doi: 10.1126/science.6828863. [DOI] [PubMed] [Google Scholar]
- Ding G. J., Ding V. D., Rodkey J. A., Bennett C. D., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. DNA sequence analysis of a Yb2 cDNA clone and regulation of the Yb1 and Yb2 mRNAs by phenobarbital. J Biol Chem. 1986 Jun 15;261(17):7952–7957. [PubMed] [Google Scholar]
- Guthenberg C., Morgenstern R., DePierre J. W., Mannervik B. Induction of glutathione S-transferases A, B and C in rat liver cytosol by trans-stilbene oxide. Biochim Biophys Acta. 1980 Aug 1;631(1):1–10. doi: 10.1016/0304-4165(80)90047-1. [DOI] [PubMed] [Google Scholar]
- Guthenberg C., Warholm M., Rane A., Mannervik B. Two distinct forms of glutathione transferase from human foetal liver. Purification and comparison with isoenzymes isolated from adult liver and placenta. Biochem J. 1986 May 1;235(3):741–745. doi: 10.1042/bj2350741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma H., Listowsky I. Identification of Yb-glutathione-S-transferase as a major rat liver protein labeled with dexamethasone 21-methanesulfonate. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7165–7169. doi: 10.1073/pnas.82.21.7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa M., Osada S., Koyama Y., Suzuki T., Hirom P. C., Diccianni M. B., Morimura S., Muramatsu M. SF-B that binds to a negative element in glutathione transferase P gene is similar or identical to trans-activator LAP/IL6-DBP. Biochem Biophys Res Commun. 1991 Aug 30;179(1):293–300. doi: 10.1016/0006-291x(91)91368-m. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B., Ketterer B., Mannervik B. Glutathione transferases: nomenclature. Biochem Pharmacol. 1984 Aug 15;33(16):2539–2540. doi: 10.1016/0006-2952(84)90621-x. [DOI] [PubMed] [Google Scholar]
- Jensson H., Eriksson L. C., Mannervik B. Selective expression of glutathione transferase isoenzymes in chemically induced preneoplastic rat hepatocyte nodules. FEBS Lett. 1985 Jul 22;187(1):115–120. doi: 10.1016/0014-5793(85)81225-4. [DOI] [PubMed] [Google Scholar]
- Kamimura S., Gaal K., Britton R. S., Bacon B. R., Triadafilopoulos G., Tsukamoto H. Increased 4-hydroxynonenal levels in experimental alcoholic liver disease: association of lipid peroxidation with liver fibrogenesis. Hepatology. 1992 Aug;16(2):448–453. doi: 10.1002/hep.1840160225. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Akira S., Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992 Oct 23;258(5082):593–597. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- Kitahara A., Satoh K., Nishimura K., Ishikawa T., Ruike K., Sato K., Tsuda H., Ito N. Changes in molecular forms of rat hepatic glutathione S-transferase during chemical hepatocarcinogenesis. Cancer Res. 1984 Jun;44(6):2698–2703. [PubMed] [Google Scholar]
- Lee S. J., Boyer T. D. The effect of hepatic regeneration on the expression of the glutathione S-transferases. Biochem J. 1993 Jul 1;293(Pt 1):137–142. doi: 10.1042/bj2930137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J. J., Bissell D. M., Friedman S. L., Roll F. J. Collagen measured in primary cultures of normal rat hepatocytes derives from lipocytes within the monolayer. J Clin Invest. 1988 Aug;82(2):450–459. doi: 10.1172/JCI113618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J. J., Friedman S. L. Parenchymal and nonparenchymal cell interactions in the liver. Semin Liver Dis. 1993 Feb;13(1):13–20. doi: 10.1055/s-2007-1007334. [DOI] [PubMed] [Google Scholar]
- Maher J. J., McGuire R. F. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990 Nov;86(5):1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Coles B., Pemble S. E., Gilmore K. S., Fraser G. M., Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochem J. 1991 Mar 1;274(Pt 2):409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimura S., Okuda A., Sakai M., Imagawa M., Muramatsu M. Analysis of glutathione transferase P gene regulation with liver cells in primary culture. Cell Growth Differ. 1992 Oct;3(10):685–691. [PubMed] [Google Scholar]
- Pickett C. B., Lu A. Y. Glutathione S-transferases: gene structure, regulation, and biological function. Annu Rev Biochem. 1989;58:743–764. doi: 10.1146/annurev.bi.58.070189.003523. [DOI] [PubMed] [Google Scholar]
- Pickett C. B., Telakowski-Hopkins C. A., Ding G. J., Argenbright L., Lu A. Y. Rat liver glutathione S-transferases. Complete nucleotide sequence of a glutathione S-transferase mRNA and the regulation of the Ya, Yb, and Yc mRNAs by 3-methylcholanthrene and phenobarbital. J Biol Chem. 1984 Apr 25;259(8):5182–5188. [PubMed] [Google Scholar]
- Redick J. A., Jakoby W. B., Baron J. Immunohistochemical localization of glutathione S-transferases in livers of untreated rats. J Biol Chem. 1982 Dec 25;257(24):15200–15203. [PubMed] [Google Scholar]
- Rushmore T. H., Pickett C. B. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990 Aug 25;265(24):14648–14653. [PubMed] [Google Scholar]
- Sakai M., Okuda A., Muramatsu M. Multiple regulatory elements and phorbol 12-O-tetradecanoate 13-acetate responsiveness of the rat placental glutathione transferase gene. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9456–9460. doi: 10.1073/pnas.85.24.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K. Glutathione transferases as markers of preneoplasia and neoplasia. Adv Cancer Res. 1989;52:205–255. doi: 10.1016/s0065-230x(08)60214-6. [DOI] [PubMed] [Google Scholar]
- Senjo M., Ishibashi T., Imai Y. Purification and characterization of cytosolic liver protein facilitating heme transport into apocytochrome b5 from mitochondria. Evidence for identifying the heme transfer protein as belonging to a group of glutathione S-transferases. J Biol Chem. 1985 Aug 5;260(16):9191–9196. [PubMed] [Google Scholar]
- Steinberg P., Schramm H., Schladt L., Robertson L. W., Thomas H., Oesch F. The distribution, induction and isoenzyme profile of glutathione S-transferase and glutathione peroxidase in isolated rat liver parenchymal, Kupffer and endothelial cells. Biochem J. 1989 Dec 15;264(3):737–744. doi: 10.1042/bj2640737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suguoka Y., Kano T., Okuda A., Sakai M., Kitagawa T., Muramatsu M. Cloning and the nucleotide sequence of rat glutathione S-transferase P cDNA. Nucleic Acids Res. 1985 Sep 11;13(17):6049–6057. doi: 10.1093/nar/13.17.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee L. B., Gilmore K. S., Meyer D. J., Ketterer B., Vandenberghe Y., Yeoh G. C. Expression of glutathione S-transferase during rat liver development. Biochem J. 1992 Feb 15;282(Pt 1):209–218. doi: 10.1042/bj2820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping E., Ketterer B. The influence of soluble binding proteins on lipophile transport and metabolism in hepatocytes. Biochem J. 1981 May 1;195(2):441–452. doi: 10.1042/bj1950441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng S. C., Smuckler E. A., Stern R. Types of collagen synthesized by normal rat liver hepatocytes in primary culture. Hepatology. 1983 Nov-Dec;3(6):955–963. doi: 10.1002/hep.1840030613. [DOI] [PubMed] [Google Scholar]
- Vandenberghe Y., Glaise D., Meyer D. J., Guillouzo A., Ketterer B. Glutathione transferase isoenzymes in cultured rat hepatocytes. Biochem Pharmacol. 1988 Jun 15;37(12):2482–2485. doi: 10.1016/0006-2952(88)90378-4. [DOI] [PubMed] [Google Scholar]
- Vandenberghe Y., Morel F., Foriers A., Ketterer B., Vercruysse A., Guillouzo A., Rogiers V. Effect of phenobarbital on the expression of glutathione S-transferase isoenzymes in cultured rat hepatocytes. FEBS Lett. 1989 Jul 17;251(1-2):59–64. doi: 10.1016/0014-5793(89)81428-0. [DOI] [PubMed] [Google Scholar]
- Vandenberghe Y., Tee L., Morel F., Rogiers V., Guillouzo A., Yeoh G. Regulation of glutathione S-transferase gene expression by phenobarbital in cultured adult rat hepatocytes. FEBS Lett. 1991 Jun 17;284(1):103–108. doi: 10.1016/0014-5793(91)80772-u. [DOI] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Schwartz S. M., Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Williams L. T., Schwartz S. M., Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988 Sep;82(3):1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Goresky C. A., Sellin J., Gatmaitan Z., Arias I. M. Role of ligandin in transfer of bilirubin from plasma into liver. Am J Physiol. 1979 Jun;236(6):E638–E648. doi: 10.1152/ajpendo.1979.236.6.E638. [DOI] [PubMed] [Google Scholar]