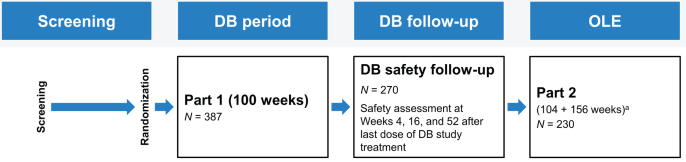
Fig. 1.
Overview of study design for the double-blind and OLE stages. aIn addition to the initial 2 years in OLE, participants were given the option to continue receiving open-label gantenerumab treatment until the end of 2020. Participants who discontinued study drug at any time during OLE or who completed the first 2 years of OLE only were asked to complete follow-up visits at 4 and 16 weeks from their last dose. Participants who received open-label gantenerumab for an additional 3 years beyond the initial 2 years of OLE were given the option of enrolling in an open-label rollover study (Open RoAD [NCT04339413]) aimed at evaluating the safety and tolerability of long-term administration of gantenerumab. Participants who rolled over to Open RoAD had one follow-up visit 4 weeks following the last dose, and those who did not had follow-up visits at 4 and 16 weeks from their last dose. DB, double-blind; OLE, open-label extension.