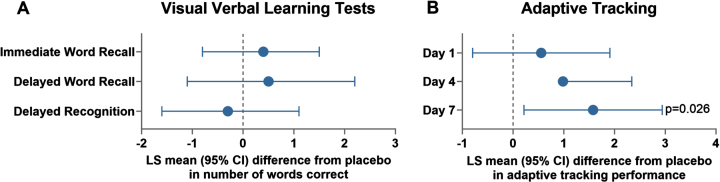
Fig. 3.
Effects of repeat, once-daily oral doses of clenbuterol (80μg) in healthy subjects in immediate word recall (A) and adaptive tracking (B) in part B. LS, Least Square; CI, Confidence Interval. Data for the cohort of 14-15 healthy volunteers in Part B who received placebo and clenbuterol (up-titrated over the dosing period from 20μg on Day 1, 40μg on Day 2 and 80μg on Days 3–7) in a 7-day crossover are presented. LS mean (95% CI) differences between clenbuterol and placebo are plotted for repeat observations at 1, 2, and 8 h after dosing on Days 1, 4, and 7 for the number of correct responses for the immediate word recall (trial 1), delayed word recall and delayed word recognition (B), and repeat observations over the first 4 h after dosing on Days 1, 4, and 7 for adaptive tracking (B).