Abstract
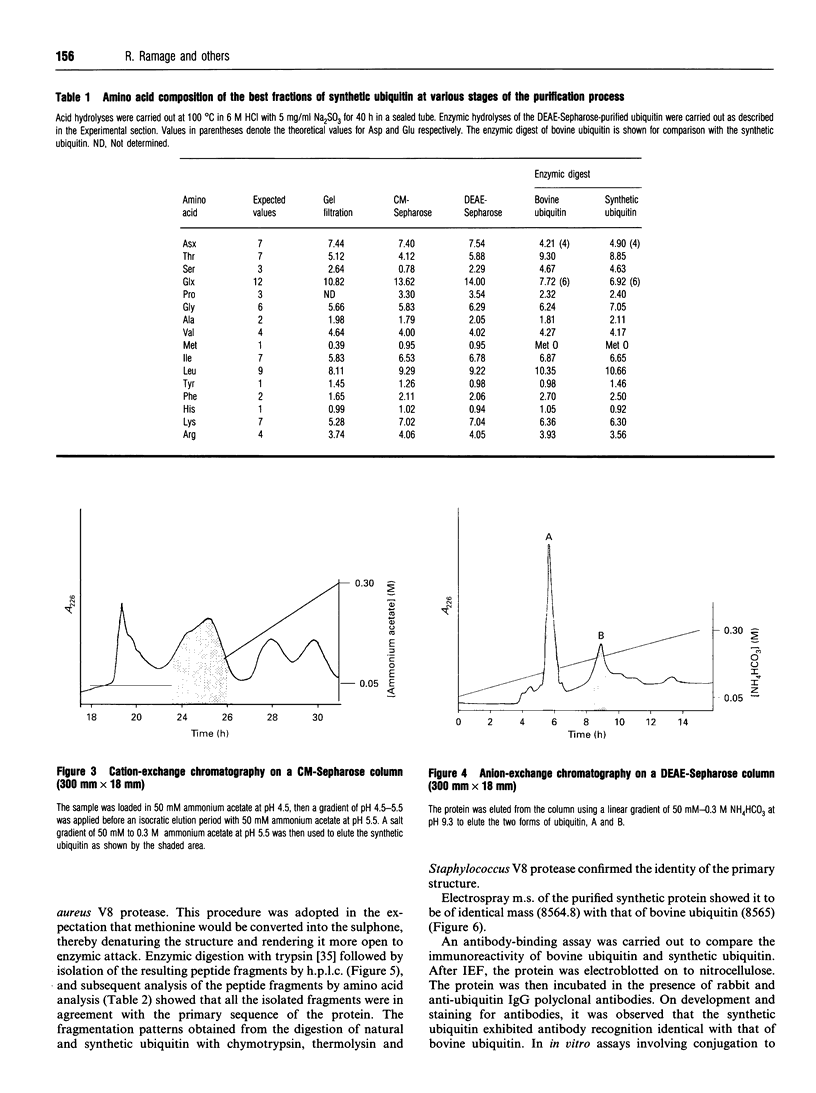
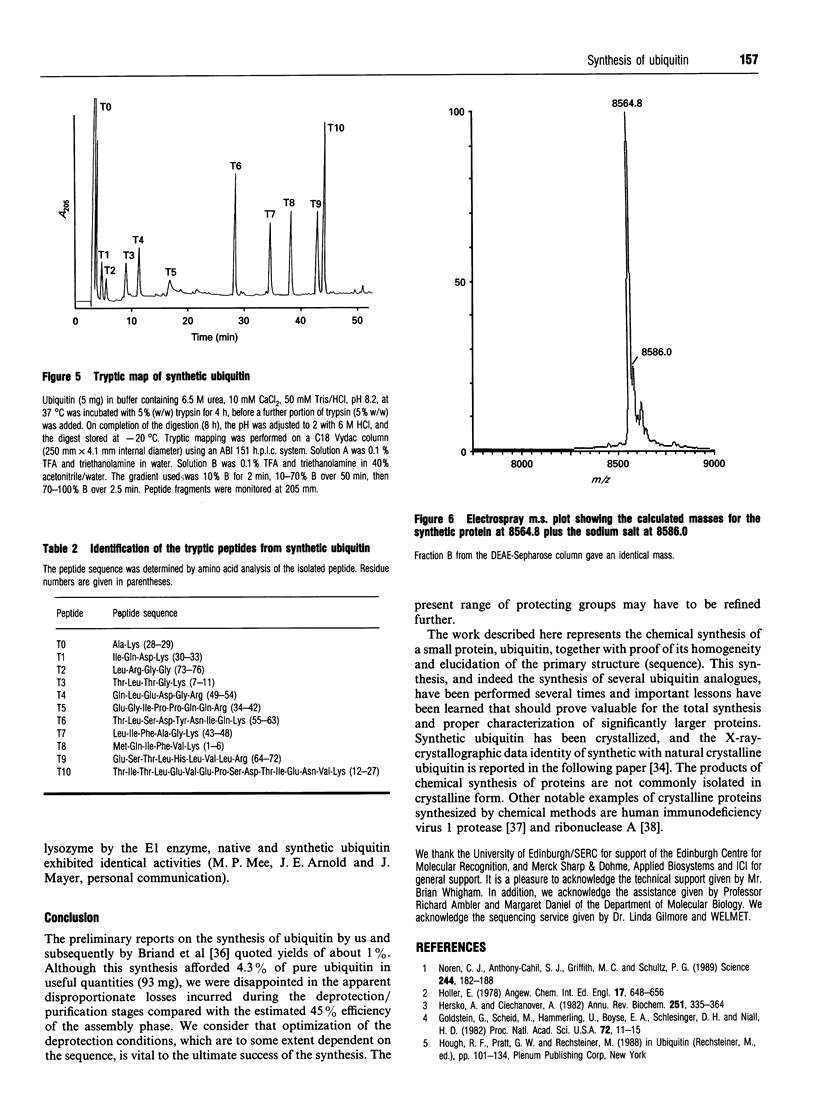
The small protein ubiquitin (76 amino acids) has been synthesized under optimized conditions by Merrifield solid-phase methodology using the N alpha-Fmoc protecting group. The crude polypeptide mixture was purified to homogeneity by gel filtration, dialysis and a combination of cation- and anion-exchange chromatography to yield ubiquitin. Amino acid analysis, enzymic digestion and sequencing by automated Edman degradation were used to authenticate the primary structure. Isoelectric focusing and m.s. were used to demonstrate that the final product was greater than 98% pure with a final yield of 93 mg (4.3%) from a single synthesis on a 0.25 nmol scale.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexeev D., Bury S. M., Turner M. A., Ogunjobi O. M., Muir T. W., Ramage R., Sawyer L. Synthetic, structural and biological studies of the ubiquitin system: chemically synthesized and native ubiquitin fold into identical three-dimensional structures. Biochem J. 1994 Apr 1;299(Pt 1):159–163. doi: 10.1042/bj2990159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamezai S., Banez M. A., Breslow E. Structural and functional changes associated with modification of the ubiquitin methionine. Biochemistry. 1990 Jun 5;29(22):5389–5396. doi: 10.1021/bi00474a026. [DOI] [PubMed] [Google Scholar]
- Briand J. P., Van Dorsselaer A., Raboy B., Muller S. Total chemical synthesis of ubiquitin using BOP reagent: biochemical and immunochemical properties of the purified synthetic product. Pept Res. 1989 Nov-Dec;2(6):381–388. [PubMed] [Google Scholar]
- Cherney B. W., Chaudhry B., Bhatia K., Butt T. R., Smulson M. Expression and mutagenesis of human poly(ADP-ribose) polymerase as a ubiquitin fusion protein from Escherichia coli. Biochemistry. 1991 Oct 29;30(43):10420–10427. doi: 10.1021/bi00107a009. [DOI] [PubMed] [Google Scholar]
- Cox M. J., Shapira R., Wilkinson K. D. Tryptic peptide mapping of ubiquitin and derivatives using reverse-phase high performance liquid chromatography. Anal Biochem. 1986 Apr;154(1):345–352. doi: 10.1016/0003-2697(86)90535-x. [DOI] [PubMed] [Google Scholar]
- Di Stefano D. L., Wand A. J. Two-dimensional 1H NMR study of human ubiquitin: a main chain directed assignment and structure analysis. Biochemistry. 1987 Nov 17;26(23):7272–7281. doi: 10.1021/bi00397a012. [DOI] [PubMed] [Google Scholar]
- Finley D., Bartel B., Varshavsky A. The tails of ubiquitin precursors are ribosomal proteins whose fusion to ubiquitin facilitates ribosome biogenesis. Nature. 1989 Mar 30;338(6214):394–401. doi: 10.1038/338394a0. [DOI] [PubMed] [Google Scholar]
- Finley D., Ciechanover A., Varshavsky A. Thermolability of ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):43–55. doi: 10.1016/0092-8674(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Scheid M., Hammerling U., Schlesinger D. H., Niall H. D., Boyse E. A. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):11–15. doi: 10.1073/pnas.72.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Holler E. Protein biosynthesis: the codon-specific activation of amino acids. Angew Chem Int Ed Engl. 1978 Sep;17(9):648–656. doi: 10.1002/anie.197806481. [DOI] [PubMed] [Google Scholar]
- Hough R., Pratt G., Rechsteiner M. Ubiquitin-lysozyme conjugates. Identification and characterization of an ATP-dependent protease from rabbit reticulocyte lysates. J Biol Chem. 1986 Feb 15;261(5):2400–2408. [PubMed] [Google Scholar]
- Hunt L. T., Dayhoff M. O. Amino-terminal sequence identity of ubiquitin and the nonhistone component of nuclear protein A24. Biochem Biophys Res Commun. 1977 Jan 24;74(2):650–655. doi: 10.1016/0006-291x(77)90352-7. [DOI] [PubMed] [Google Scholar]
- Miller M., Schneider J., Sathyanarayana B. K., Toth M. V., Marshall G. R., Clawson L., Selk L., Kent S. B., Wlodawer A. Structure of complex of synthetic HIV-1 protease with a substrate-based inhibitor at 2.3 A resolution. Science. 1989 Dec 1;246(4934):1149–1152. doi: 10.1126/science.2686029. [DOI] [PubMed] [Google Scholar]
- Monia B. P., Ecker D. J., Jonnalagadda S., Marsh J., Gotlib L., Butt T. R., Crooke S. T. Gene synthesis, expression, and processing of human ubiquitin carboxyl extension proteins. J Biol Chem. 1989 Mar 5;264(7):4093–4103. [PubMed] [Google Scholar]
- Mori H., Kondo J., Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987 Mar 27;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- Nickel B. E., Davie J. R. Structure of polyubiquitinated histone H2A. Biochemistry. 1989 Feb 7;28(3):964–968. doi: 10.1021/bi00429a007. [DOI] [PubMed] [Google Scholar]
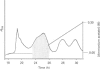
- Noren C. J., Anthony-Cahill S. J., Griffith M. C., Schultz P. G. A general method for site-specific incorporation of unnatural amino acids into proteins. Science. 1989 Apr 14;244(4901):182–188. doi: 10.1126/science.2649980. [DOI] [PubMed] [Google Scholar]
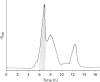
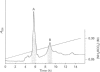
- Ogunjobi O., Ramage R. Ubiquitin: preparative chemical synthesis, purification and characterization. Biochem Soc Trans. 1990 Dec;18(6):1322–1323. doi: 10.1042/bst0181322. [DOI] [PubMed] [Google Scholar]
- Redman K. L., Rechsteiner M. Identification of the long ubiquitin extension as ribosomal protein S27a. Nature. 1989 Mar 30;338(6214):438–440. doi: 10.1038/338438a0. [DOI] [PubMed] [Google Scholar]
- Salvesen G., Lloyd C., Farley D. cDNA encoding a human homolog of yeast ubiquitin 1. Nucleic Acids Res. 1987 Jul 10;15(13):5485–5485. doi: 10.1093/nar/15.13.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman M., Bond M. W., Gallatin W. M., St John T., Smith H. T., Fried V. A., Weissman I. L. Cell surface molecule associated with lymphocyte homing is a ubiquitinated branched-chain glycoprotein. Science. 1986 Feb 21;231(4740):823–829. doi: 10.1126/science.3003913. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar S., Bugg C. E., Wilkinson K. D., Cook W. J. Three-dimensional structure of ubiquitin at 2.8 A resolution. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3582–3585. doi: 10.1073/pnas.82.11.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. S. p-alkoxybenzyl alcohol resin and p-alkoxybenzyloxycarbonylhydrazide resin for solid phase synthesis of protected peptide fragments. J Am Chem Soc. 1973 Feb 21;95(4):1328–1333. doi: 10.1021/ja00785a602. [DOI] [PubMed] [Google Scholar]
- Weber P. L., Brown S. C., Mueller L. Sequential 1H NMR assignments and secondary structure identification of human ubiquitin. Biochemistry. 1987 Nov 17;26(23):7282–7290. doi: 10.1021/bi00397a013. [DOI] [PubMed] [Google Scholar]
- Yajima H., Fujii N. Totally synthetic crystalline ribonuclease A. Biopolymers. 1981 Sep;20(9):1859–1867. doi: 10.1002/bip.1981.360200910. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]