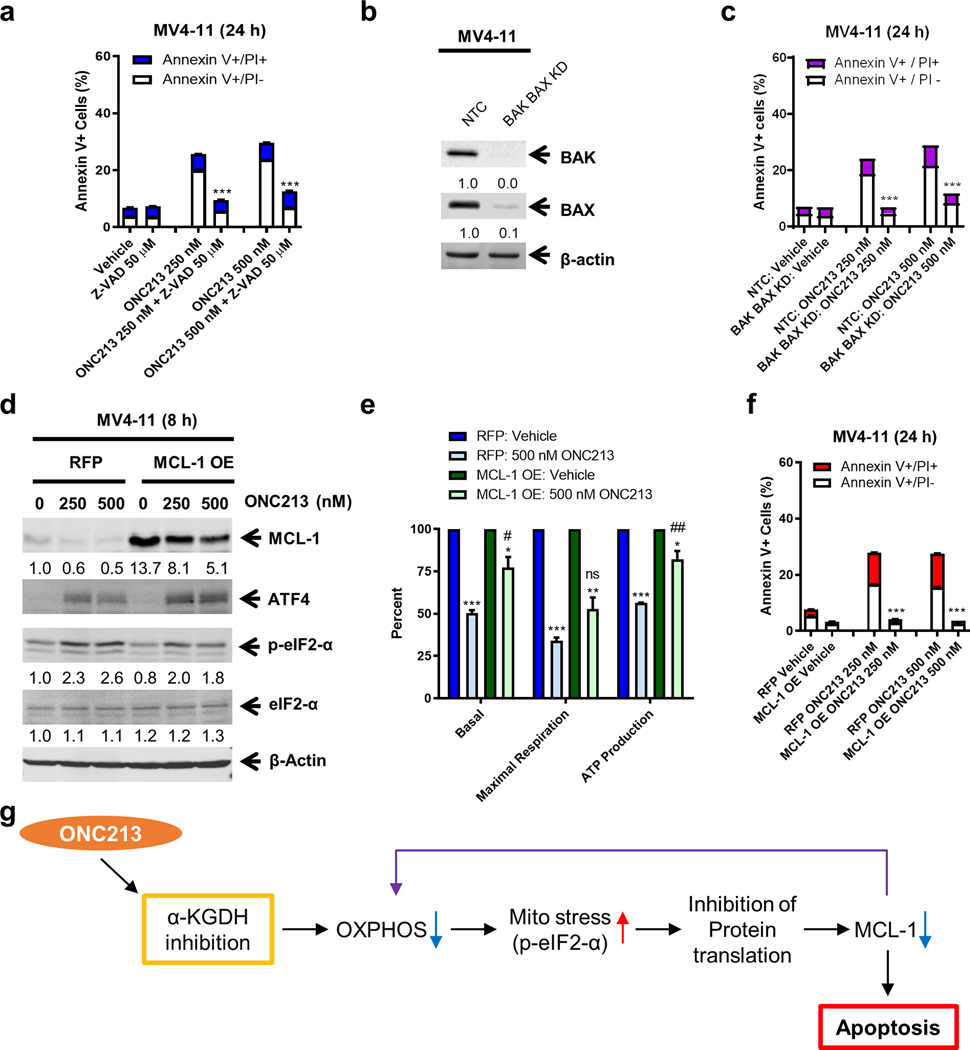
Fig. 7. Relationship between MCL-1 and ONC213 sensitivity.
a. MV4–11 cells were treated with vehicle or ONC213 in the presence or absence of Z-VAD-FMK for 24 h and then analyzed by Annexin V-FITC/PI staining and flow cytometry analysis. *** P<0.001 compared to ONC213 treatment. b. Lentiviral shRNA double-knockdown of BAK and BAX was performed in MV4–11 cells (designated as BAK/BAX KD cells). Non-template negative-control shRNA was used as the control (designated as NTC cells). BAK and BAX double knockdown was confirmed by western blot. c. BAK/BAX KD and NTC cells were treated with vehicle or ONC213 for 24 h and then analyzed by Annexin V-FITC/PI staining and flow cytometry analysis. *** P<0.001. d. Lentiviral overexpression of RFP (red fluorescent protein) and MCL-1 (designated MCL-1 OE) was performed in MV4–11 cells. RFP control and MCL-1 OE cells were treated with vehicle or ONC213 for 8 h and then whole-cell lysates were analyzed by western blot and probed with the indicated antibodies. The fold changes for the MCL-1 densitometry measurements, normalized to β-actin and then compared to vehicle control, are indicated. e. The RFP control and MCL-1 OE cells were treated with vehicle or ONC213 for 8 h. Basal OCR, maximal respiration, and ATP production were measured using a Seahorse flux analyzer. * P<0.05; ** P <0.01; *** P<0.001 compared to vehicle control. ns, not significant; # P<0.05; ## P <0.01 compared to RFP treated with 500 nM ONC213. f. The RFP control and MCL-1 OE cells were treated with vehicle or ONC213 for 24 h and then analyzed by Annexin V-FITC/PI staining and flow cytometry analysis. Results are shown as mean Annexin V+ cells ± SEM. *** P<0.001 compared to RFP under the same treatment conditions. g. Proposed mechanism of action of ONC213. ONC213 inhibits α-KGDH, leading to reduced OXPHOS and mitochondrial stress (increase of eIF2α phosphorylation) in AML cells. This stress response leads to inhibition of protein translation, resulting in decrease of a key antiapoptotic protein, MCL-1. Decrease of MCL-1 results in further decrease of OXPHOS, leading to death of AML cells that are reliant on OXPHOS.