Abstract
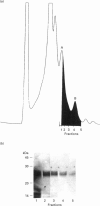
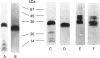
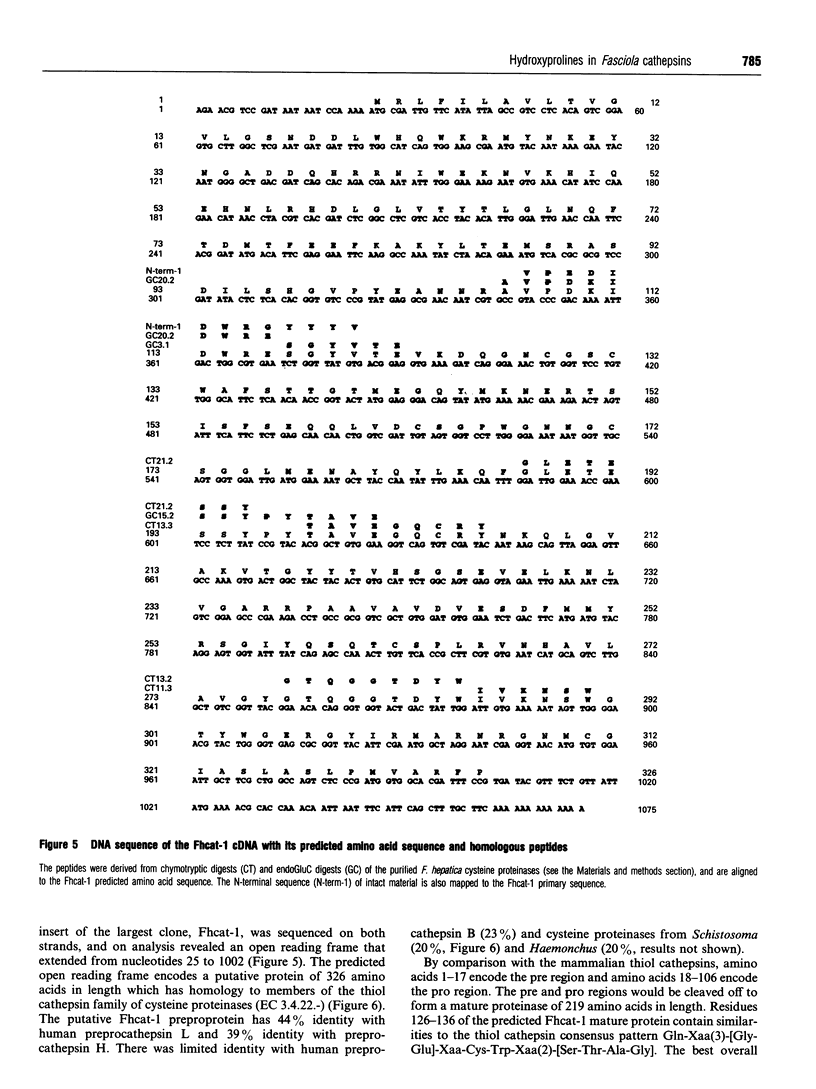
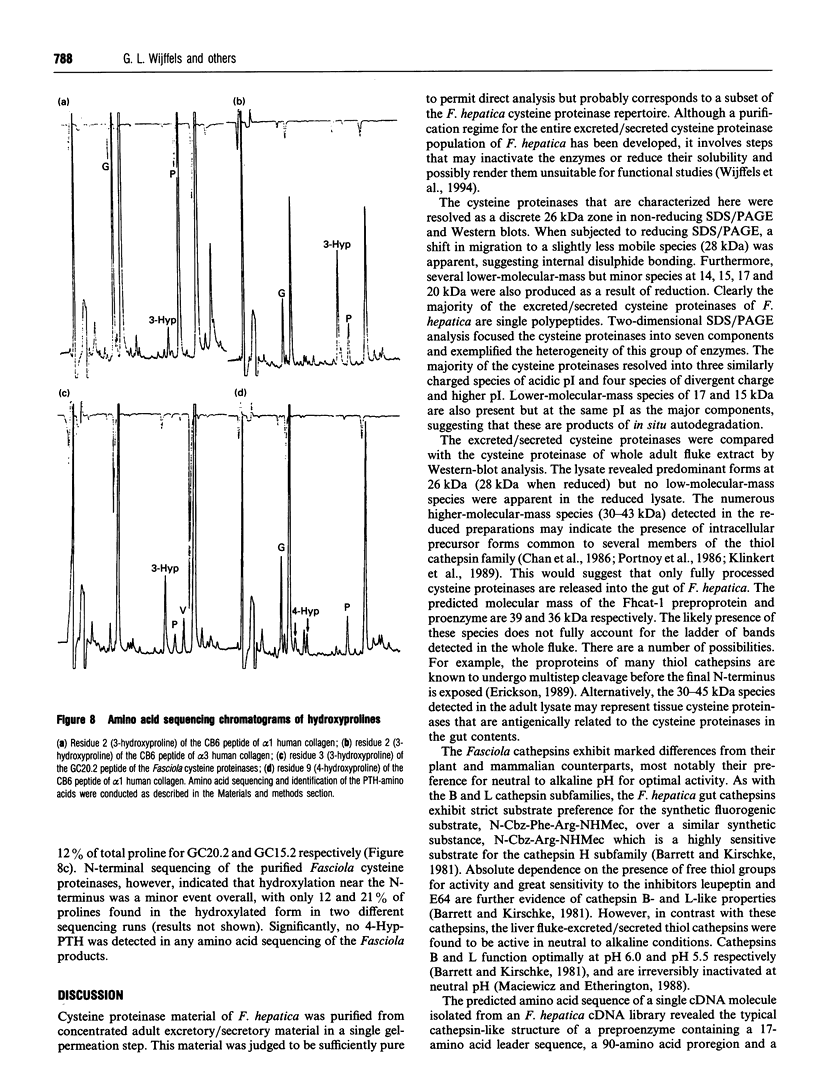
The cysteine proteinases synthesized by the adult stage of the trematode Fasciola hepatica were found to be a very heterogeneous group of proteins as demonstrated by one- and two-dimensional gel analyses. N-terminal amino acid sequencing indicated the presence of at least two distinct gene products among the secreted cysteine proteinases. Enzymic studies and peptide sequence analysis of the excreted/secreted cysteine proteinases suggested a close relationship to the plant thiol cathepsins and the mammalian cathepsin L subfamily. The cloning of a representative cDNA for a putative Fasciola cathepsin confirmed similarities to the cathepsin L subfamily but revealed low identity with the cathepsin-like proteinases of the related trematode, Schistosoma, nematode cathepsins and the mammalian cathepsin B subfamily. Furthermore, peptide and protein sequencing revealed the modification of certain highly conserved prolines to unusual 3-hydroxyproline derivatives. This is the first report of modified prolines in any proteinase. This finding, as well as the high activities of these cathepsins at neutral to alkaline pH values, raises a number of questions as to the physiological function of these thiol cathepsins and their interaction with host tissues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J. 1980 Jun 1;187(3):909–912. doi: 10.1042/bj1870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Baylis H. A., Megson A., Mottram J. C., Hall R. Characterisation of a gene for a cysteine protease from Theileria annulata. Mol Biochem Parasitol. 1992 Aug;54(1):105–107. doi: 10.1016/0166-6851(92)90100-x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., San Segundo B., McCormick M. B., Steiner D. F. Nucleotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7721–7725. doi: 10.1073/pnas.83.20.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman C. B., Mitchell G. F. Proteolytic cleavage of immunoglobulin by enzymes released by Fasciola hepatica. Vet Parasitol. 1982 Nov;11(2-3):165–178. doi: 10.1016/0304-4017(82)90039-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cole W. G., Chan D. Analysis of the heterogeneity of human collagens by two-dimensional polyacrylamide-gel electrophoresis. Biochem J. 1981 Aug 1;197(2):377–383. doi: 10.1042/bj1970377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. N., Pratt D., Hageman R., Boisvenue R. J. Molecular cloning and primary sequence of a cysteine protease expressed by Haemonchus contortus adult worms. Mol Biochem Parasitol. 1990 Jun;41(1):25–34. doi: 10.1016/0166-6851(90)90093-2. [DOI] [PubMed] [Google Scholar]
- Dalton J. P., Heffernan M. Thiol proteases released in vitro by Fasciola hepatica. Mol Biochem Parasitol. 1989 Jun 15;35(2):161–166. doi: 10.1016/0166-6851(89)90118-7. [DOI] [PubMed] [Google Scholar]
- Delaissé J. M., Ledent P., Vaes G. Collagenolytic cysteine proteinases of bone tissue. Cathepsin B, (pro)cathepsin L and a cathepsin L-like 70 kDa proteinase. Biochem J. 1991 Oct 1;279(Pt 1):167–174. doi: 10.1042/bj2790167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin A. E., Mills A. A., Harth G., McKerrow J. H., Craik C. S. The sequence, organization, and expression of the major cysteine protease (cruzain) from Trypanosoma cruzi. J Biol Chem. 1992 Apr 15;267(11):7411–7420. [PubMed] [Google Scholar]
- Erickson A. H. Biosynthesis of lysosomal endopeptidases. J Cell Biochem. 1989 May;40(1):31–41. doi: 10.1002/jcb.240400104. [DOI] [PubMed] [Google Scholar]
- Fietzek P. P., Rexrodt F. W., Wendt P., Stark M., Kühn K. The covalent structure of collagen. Amino-acid sequence of peptide 1-CB6-C2. Eur J Biochem. 1972 Oct 17;30(1):163–168. doi: 10.1111/j.1432-1033.1972.tb02083.x. [DOI] [PubMed] [Google Scholar]
- Halton D. W. Observations on the nutrition of digenetic trematodes. Parasitology. 1967 Nov;57(4):639–660. doi: 10.1017/s003118200007311x. [DOI] [PubMed] [Google Scholar]
- Klinkert M. Q., Felleisen R., Link G., Ruppel A., Beck E. Primary structures of Sm31/32 diagnostic proteins of Schistosoma mansoni and their identification as proteases. Mol Biochem Parasitol. 1989 Mar 1;33(2):113–122. doi: 10.1016/0166-6851(89)90025-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maciewicz R. A., Etherington D. J. A comparison of four cathepsins (B, L, N and S) with collagenolytic activity from rabbit spleen. Biochem J. 1988 Dec 1;256(2):433–440. doi: 10.1042/bj2560433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki J., Yanagisawa T. Demonstration of carboxyl and thiol protease activities in adult Schistosoma mansoni, Dirofilaria immitis, Angiostrongylus cantonensis and Ascaris suum. J Helminthol. 1986 Mar;60(1):31–37. doi: 10.1017/s0022149x00008191. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Walker J. E., Northrop F. D. The N-terminal amino acid sequences of the heavy and light chains of human cathepsin L. Relationship to a cDNA clone for a major cysteine proteinase from a mouse macrophage cell line. Biochem J. 1986 Dec 1;240(2):373–377. doi: 10.1042/bj2400373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerrow J. H., Doenhoff M. J. Schistosome proteases. Parasitol Today. 1988 Dec;4(12):334–340. doi: 10.1016/0169-4758(88)90002-6. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Musil D., Zucic D., Turk D., Engh R. A., Mayr I., Huber R., Popovic T., Turk V., Towatari T., Katunuma N. The refined 2.15 A X-ray crystal structure of human liver cathepsin B: the structural basis for its specificity. EMBO J. 1991 Sep;10(9):2321–2330. doi: 10.1002/j.1460-2075.1991.tb07771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Tiltman K. J., Recklies A. D., Stoker T. A. Differences in secretion of the proteinase cathepsin B at the edges of human breast carcinomas and fibroadenomas. Nature. 1978 Jun 15;273(5663):545–547. doi: 10.1038/273545a0. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Erickson A. H., Kochan J., Ravetch J. V., Unkeless J. C. Cloning and characterization of a mouse cysteine proteinase. J Biol Chem. 1986 Nov 5;261(31):14697–14703. [PubMed] [Google Scholar]
- Pratt D., Armes L. G., Hageman R., Reynolds V., Boisvenue R. J., Cox G. N. Cloning and sequence comparisons of four distinct cysteine proteases expressed by Haemonchus contortus adult worms. Mol Biochem Parasitol. 1992 Apr;51(2):209–218. doi: 10.1016/0166-6851(92)90071-q. [DOI] [PubMed] [Google Scholar]
- Ray C., McKerrow J. H. Gut-specific and developmental expression of a Caenorhabditis elegans cysteine protease gene. Mol Biochem Parasitol. 1992 Apr;51(2):239–249. doi: 10.1016/0166-6851(92)90074-t. [DOI] [PubMed] [Google Scholar]
- Rege A. A., Herrera P. R., Lopez M., Dresden M. H. Isolation and characterization of a cysteine proteinase from Fasciola hepatica adult worms. Mol Biochem Parasitol. 1989 Jun 1;35(1):89–95. doi: 10.1016/0166-6851(89)90146-1. [DOI] [PubMed] [Google Scholar]
- Rege A. A., Wang W., Dresden M. H. Cysteine proteinases from Schistosoma haematobium adult worms. J Parasitol. 1992 Feb;78(1):16–23. [PubMed] [Google Scholar]
- Ritonja A., Rowan A. D., Buttle D. J., Rawlings N. D., Turk V., Barrett A. J. Stem bromelain: amino acid sequence and implications for weak binding of cystatin. FEBS Lett. 1989 Apr 24;247(2):419–424. doi: 10.1016/0014-5793(89)81383-3. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. J., Nelson R. G. Isolation and characterization of a cysteine proteinase gene of Plasmodium falciparum. Mol Biochem Parasitol. 1992 Mar;51(1):143–152. doi: 10.1016/0166-6851(92)90209-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticher L., Hofsteenge J., Milani A., Neuhaus J. M., Meins F., Jr Vacuolar chitinases of tobacco: a new class of hydroxyproline-containing proteins. Science. 1992 Jul 31;257(5070):655–657. doi: 10.1126/science.1496378. [DOI] [PubMed] [Google Scholar]
- Tannich E., Scholze H., Nickel R., Horstmann R. D. Homologous cysteine proteinases of pathogenic and nonpathogenic Entamoeba histolytica. Differences in structure and expression. J Biol Chem. 1991 Mar 15;266(8):4798–4803. [PubMed] [Google Scholar]
- Wada K., Takai T., Tanabe T. Amino acid sequence of chicken liver cathepsin L. Eur J Biochem. 1987 Aug 17;167(1):13–18. doi: 10.1111/j.1432-1033.1987.tb13298.x. [DOI] [PubMed] [Google Scholar]
- Wijffels G. L., Sexton J. L., Salvatore L., Pettitt J. M., Humphris D. C., Panaccio M., Spithill T. W. Primary sequence heterogeneity and tissue expression of glutathione S-transferases of Fasciola hepatica. Exp Parasitol. 1992 Feb;74(1):87–99. doi: 10.1016/0014-4894(92)90142-w. [DOI] [PubMed] [Google Scholar]
- Zussman R. A., Bauman P. M., Petruska J. C. The role of ingested hemoglobin in the nutrition of Schistosoma mansoni. J Parasitol. 1970 Feb;56(1):75–79. [PubMed] [Google Scholar]