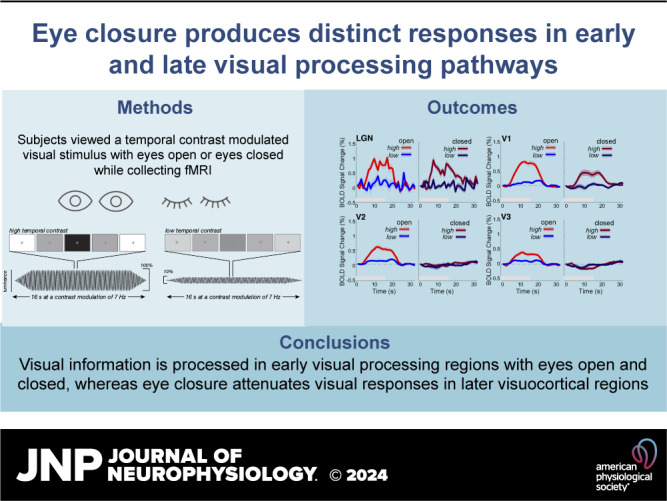
Keywords: eye closure, fMRI, luminance, LGN, visual cortex
Abstract
Closing our eyes largely shuts down our ability to see. That said, our eyelids still pass some light, allowing our visual system to coarsely process information about visual scenes, such as changes in luminance. However, the specific impact of eye closure on processing within the early visual system remains largely unknown. To understand how visual processing is modulated when eyes are shut, we used functional magnetic resonance imaging (fMRI) to measure responses to a flickering visual stimulus at high (100%) and low (10%) temporal contrasts, while participants viewed the stimuli with their eyes open or closed. Interestingly, we discovered that eye closure produced a qualitatively distinct pattern of effects across the visual thalamus and visual cortex. We found that with eyes open, low temporal contrast stimuli produced smaller responses across the lateral geniculate nucleus (LGN), primary (V1) and extrastriate visual cortex (V2). However, with eyes closed, we discovered that the LGN and V1 maintained similar blood oxygenation level-dependent (BOLD) responses as the eyes open condition, despite the suppressed visual input through the eyelid. In contrast, V2 and V3 had strongly attenuated BOLD response when eyes were closed, regardless of temporal contrast. Our findings reveal a qualitatively distinct pattern of visual processing when the eyes are closed—one that is not simply an overall attenuation but rather reflects distinct responses across visual thalamocortical networks, wherein the earliest stages of processing preserve information about stimuli but are then gated off downstream in visual cortex.
NEW & NOTEWORTHY When we close our eyes coarse luminance information is still accessible by the visual system. Using functional magnetic resonance imaging, we examined whether eyelid closure plays a unique role in visual processing. We discovered that while the LGN and V1 show equivalent responses when the eyes are open or closed, extrastriate cortex exhibited attenuated responses with eye closure. This suggests that when the eyes are closed, downstream visual processing is blind to this information.
INTRODUCTION
Light exposure during sleep has substantial effects on the brain: it can alter circadian rhythms, sleep quality, and mood (1, 2). During sleep, our eyes are closed and the eyelids function as potent filters of visual information. However, our eyelids are only partial filters and do not completely attenuate all visual information (3, 4). The eyelid has been characterized as a red-pass filter, with an estimated 6% red light spectral transmittance (3). Indeed, subjective experience with high luminance stimuli, such as during a sunny day, corroborates the idea that changes in luminance are still detectable when our eyes are closed. With partial, rather than complete, filtering properties, it follows that the visual system processes external visual information with our eyes closed, as well.
How does the visual system process information when our eyes are closed? It is possible that the filtering properties of the eyelid simply quantitatively suppress responses across visual regions, due to the attenuation of input. Alternatively, eye closure could induce qualitatively distinct changes in visual response, selectively modulating responses in specific brain networks. Although little is known about stimulus-evoked visual responses with eyes closed, resting-state functional magnetic resonance imaging (fMRI) studies have investigated spontaneous dynamics during eye closure in the absence of any visual stimulus presentation (5–7). These studies found differences in resting-state functional connectivity in attentional networks depending on whether eyes were open or closed, along with differences in activation in prefrontal cortex, parietal and frontal eye fields, and lateral geniculate nucleus (LGN). While eye closure appears to play a unique role in modulating brain responses, the impact that eye closure has on stimulus-evoked visual responses remains poorly understood.
In this study, we sought to shed light on the role that eye closure plays in modulating responses within the visual processing hierarchy. To do so, we measured fMRI blood oxygenation level-dependent (BOLD) responses within visual cortex and subcortex while participants viewed high and low-intensity visual stimuli, with their eyes open or shut. We manipulated the intensity of visual input via temporal contrast modulation, in which the luminance of uniform visual stimuli flickered rapidly between extreme whites and blacks (high temporal contrast), or between middling intensities (low temporal contrast). Indeed, previous work has shown visuocortical responses to be sensitive to changes in luminance (8). By measuring BOLD responses to high and low luminance contrast stimuli, we examined whether there is a qualitatively unique pattern of luminance responses across the visuocortical hierarchy when one’s eyes are closed, compared with when they are open.
MATERIALS AND METHODS
Participants
Data was acquired from a total of eight healthy participants (5 females, 3 males; 3 Asian, 1 Black or African American; 4 White). Participants were aged 18–35 yr, reported normal or corrected-to-normal visual acuity, and were recruited from Boston University and the surrounding community. All participants provided written informed consent before study enrollment and completed a metal screening form indicating that they had no MRI contraindications. Participants were reimbursed for their study participation.
All aspects of the study were approved by Boston University’s Institutional Review Board.
Apparatus and Stimuli
Stimuli were generated using custom software written in MATLAB (version 2019b) in conjunction with Psychtoolbox (9). Participants viewed stimuli that were back-projected onto a screen set within the MRI scanner, using a ProPIXX DLP LED (VPixx Technologies) projector system (minimum luminance: 1.2 cd/m2; maximum luminance: 2,507.9 cd/m2). Photometer measurements (model LS-100; Konica Minolta) carried out before the study were used to verify the linearity of the display [1 digital-to-analog conversion (DAC) step = 9.835 cd/m2]. These measurements were used to calculate the stimulus luminance and were acquired from the inner-facing side of the back-projection screen while positioned within the MRI scanner bore. This was done to best account for the attenuation in luminance due to back-projection screen characteristics.
During each functional run, participants fixated on a median luminance crosshair at the center of the display while shown a full-screen flickering display (17° of visual angle) with no spatial contrast (Fig. 1). The full field flicker was presented in a block design with three trial types (baseline, high, and low temporal luminance contrast), with each event lasting 16 s. In the baseline events, the full field display was a constant median luminance with no luminance modulation. During high events, the full field display flickered with an amplitude envelope of 100% around the middle luminance value. For low events, the full field display flickered with an amplitude envelope of 10% around the median luminance value. All high and low events flickered at a frequency of 7 Hz.
Figure 1.
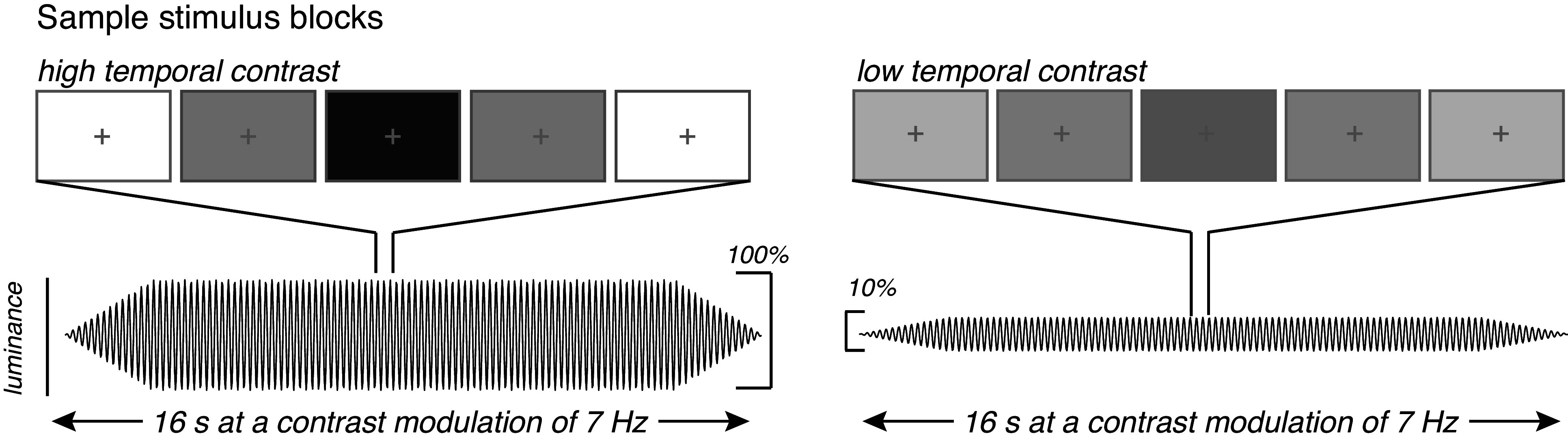
Experimental design with sample stimulus frames displaying the high temporal contrast and low temporal contrast displays. High temporal contrast flickered at 7 Hz with a luminance amplitude envelope of 100%, encompassing the maximum (255 au) and minimum (0 au) possible luminance values. The low temporal contrast events also flickered at 7 Hz with a luminance amplitude envelope of 10%, encompassing a range of luminance values between 140 au and 115 au.
Experimental Design
Subjects participated in two scan sessions, each lasting approximately two hours. The first session was dedicated to collecting anatomical images and data for population receptive field (pRF) mapping using standard techniques and stimuli (10, 11). The second session was dedicated to collecting proton-density (PD) weighted anatomical imaging and fMRI blood oxygenation level-dependent (BOLD) data across the eyes open and closed conditions, during the luminance task.
During the second experimental session, we collected three PD-weighted anatomical scans. PD-weighted anatomical imaging has previously been used to better localize the LGN (12, 13). Following the PD-weighted scans, participants completed three consecutive runs of a functional localizer. The visual stimulus for the functional localizer contained a full field flickering grating stimulus (diameter = 6.0°) with a centered circle (diameter = 0.8°). Within the centered circle, letters rapidly appeared one at a time with a new letter appearing every 200 ms. Participants were instructed to press a button whenever the letters “J” and “K” appeared within the centered circle. During the localizer blocks, the full field display alternated between a flickering grating stimulus and a full field nonflickering display at median luminance value. Participants completed 12 total blocks (6 flickering field, 6 nonflickering field) with an extra nonflickering block at the beginning of the run. At the end of each localizer run, participants were asked to report their wakefulness level.
Participants then completed the luminance flicker task. The task began and ended with a baseline event. High and low temporal contrast conditions were pseudo-randomly ordered, with all high and low events interleaved with a baseline event. Each run contained 12 events (6 high and 6 low) interspersed with 12 baseline events, lasting a total of 384 s. On each run, participants were instructed to press a button after each full breath cycle (1 inhale and 1 exhale). This button task was chosen to ensure that participants did not fall asleep and engaged with the task, while not requiring eyes to be open. For each run, participants were instructed to either keep their eyes open and fixate on the crosshair or to keep their eyes closed throughout the run. Each scan session began with an eyes-closed run, and consecutive runs alternated between open and closed conditions. We always began with the eyes closed condition to ensure we acquired a sufficient number of runs in this condition, where BOLD modulations may be lower compared with eyes-open runs. To ensure participants kept their eyes closed or open, real-time eye monitoring was carried out using an EyeLink1000, for the duration of each run. On average, we collected five runs with eyes closed and four runs with eyes open, for each subject.
MRI Data Acquisition
All neuroimaging data were acquired using a research-dedicated Siemens Prisma 3 T scanner using a Siemens 64-channel head coil. A whole brain anatomical scan was acquired using a T1-weighted multi-echo MPRAGE [1 mm isotropic voxels; field of view (FOV) = 192 × 192 × 134 mm, flip angle (FA) = 7.00°, repetition time (TR) = 2,200 ms, echo time (TE) = 1.57 ms]. Proton density (PD)-weighted anatomical scans were acquired to localize LGN (0.9 mm × 0.9 mm × 1.7 mm; TR = 2,950.0 ms; TE = 15.6 ms; FA = 180°). Functional scans were acquired using T2*-weighted in-plane simultaneous imaging (2 mm isotropic voxels; FOV = 104 × 104 × 70 mm, FA = 64.00°, TR = 1,000 ms, TE = 30 ms, SMS factor = 5, GRAPPA acceleration = 2).
Anatomical Data Analysis
T1-weighted anatomical data were analyzed using the standard “recon-all” pipeline provided by the FreeSurfer neuroimaging analysis package (14), generating cortical surface models, whole brain segmentations, and cortical parcellations. All PD-weighted scans were aligned to each subject’s anatomical space and averaged together (using AFNI’s 3dcalc).
Functional Data Analysis
Functional BOLD time-series data were first corrected for echo-planar imaging (EPI) distortions using a reverse phase-encode method implemented in FSL (15) and were then preprocessed with FS-FAST using standard motion-correction procedures, slice timing correction, and boundary-based registration between functional and anatomical spaces (16). To optimize spatial precision of experimental data, no volumetric spatial smoothing was performed (full-width half-maximum 0 mm). To achieve precise alignment of experimental data within the session, cross-run within-modality robust rigid registration was performed, using the middle time point of each run (17). BOLD time-series data were demeaned and converted to units of percent signal change. Data collected during the separate pRF mapping scans were analyzed using the analyzePRF toolbox (11). Results from the pRF model were used to manually draw labels for our regions of interest within visual cortex.
Statistical Analysis
The results from the pRF modeling were used to identify region-of-interest (ROI) labels for each cortical region before analysis. ROI labels included voxels located inside the cortical ribbon for primary visual cortex (V1) and extrastriate visual cortex (V2 and V3), which were identified using a visual area network label generated using an intrinsic functional connectivity atlas (18). Results from the pRF modeling were additionally used to select voxels with visual field eccentricity preferences less than 17 degrees visual angle away from fixation as this was the measured extent of the screen within the MRI scanner. Cortical voxels with a poor pRF model fit (r2 < 0.10) were removed from further analyses. Initial LGN labels were acquired from thalamic segmentation and parcellation in Free-Surfer for each participant. These initial labels were overlaid with the GLM results from the functional localizer and the PD-weighted scans, and only intersecting voxels were chosen for the final LGN labels and further analyses.
An event-triggered average was computed for each flickering condition (low and high) per eyelid condition and ROI. The BOLD time-series for each ROI per run was separated by the low and high trials, and all trials of a given type were averaged together. Average BOLD magnitude in response to the stimulus presentation was computed by averaging 4–16 s poststimulus onset for each trial. Two-way between-subjects ANOVA was performed to test for any main effects of temporal contrast and eye closure and any interaction of the two on average BOLD magnitude during stimulus presentation. Additional event-triggered average analysis was done with eccentricity, in which the time-series for V1/V2/V3 voxels were first separated into eccentricity bins defined by degree visual angle relative to fixation. Foveal-tuned voxels were between 0.01°–1.5°, parafoveal-tuned voxels were between 1.5°–4.0°, and peripheral-tuned voxels were between 4.0°–17.0°. An additional ANOVA was performed to test for any main effect of eccentricity on BOLD response during stimulus presentation. Multiple-comparison correction was done using Bonferroni correction of α/n at a familywise α of 0.05 where n is the number of tests performed.
RESULTS
We first examined how temporal contrast modulated thalamic and visuocortical responses, and if eye closure impacted these responses. With eyes open, LGN, V1, and V2 showed larger responses to high temporal contrast stimuli, compared with low temporal contrast stimuli (Fig. 2A). Indeed, during eyes open with high temporal contrast stimuli, all ROIs had significantly elevated BOLD responses [LGN: t(7) = 3.27, P = 0.006; V1: t(7) = 5.16, P < 0.0001; V2: t(7) = 3.40, P = 0.005; V3: t(7) = 2.54, P = 0.019), though the significant response in V3 did not survive multiple comparisons correction. When the participants closed their eyes, however, LGN and V1 maintained their stronger responses to higher contrast stimuli [LGN: F(1,31) = 4.31, P = 0.047; V1: F(1,31) = 11.05, P = 0.002], which did not differ from their eyes closed conditions [LGN: F(1,31) = 0.02, P = 0.975; V1: F(1,31) = 1.74, P = 0.20]. In other words, while responses in LGN and V1 were significantly modulated by temporal contrast, they were completely unaffected by eye closure, despite the profound suppression of visual input from the eyelid.
Figure 2.
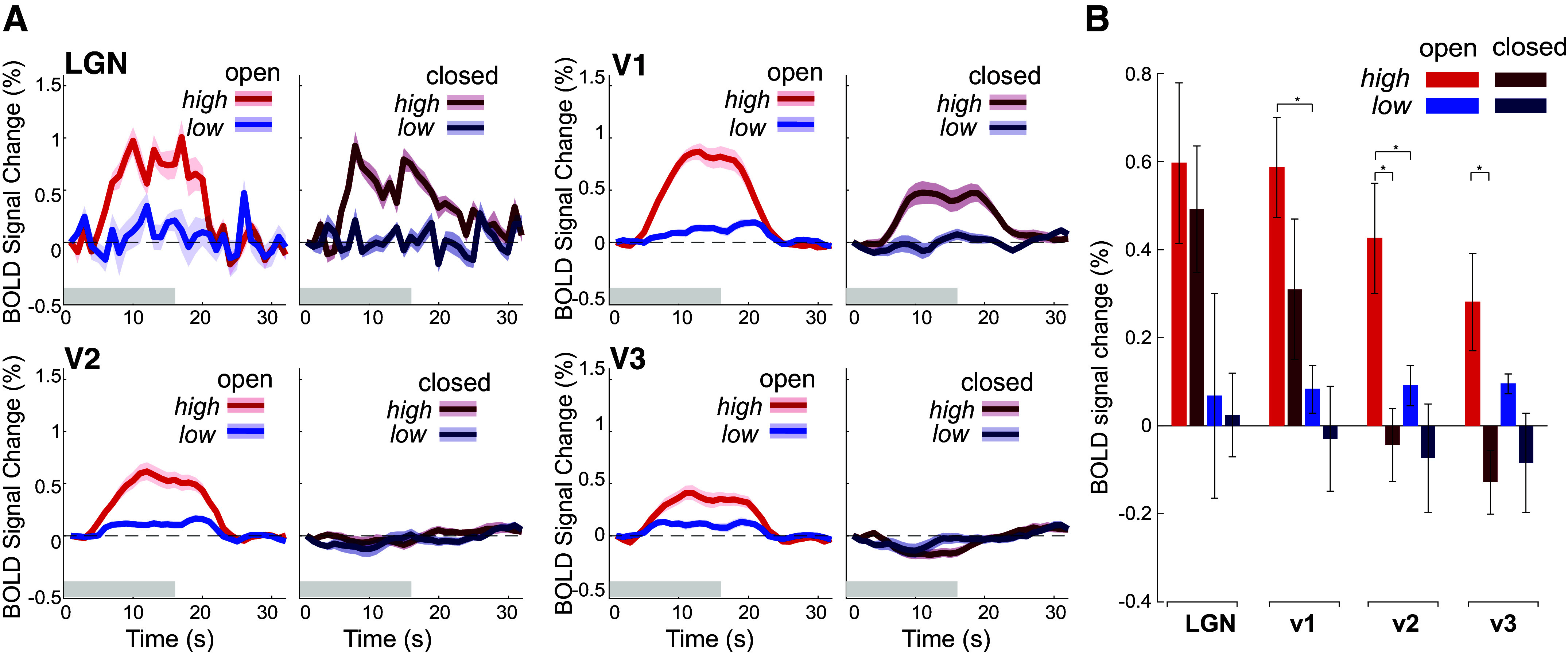
Eye closure has minimal effect on visual responses in LGN and V1, while suppressing responses in V2 and V3. A: event-triggered average for luminance task across ROI and eye condition. Across LGN, V1, and V2, during eyes open runs, high temporal contrast stimuli elicit a greater BOLD response than with low temporal contrast stimuli. Although there is no effect of temporal contrast in V3, BOLD response increases regardless of the stimulus temporal contrast. During eye closure, BOLD responses in LGN and V1 during the high temporal contrast stimuli elicit a similar BOLD response as during eyes open runs. With eye closure, V2 and V3 have strongly attenuated BOLD regardless of temporal contrast. Red plots indicate high temporal contrast trials and blue indicates the low temporal contrast trials. The gray bar indicates 16-s period of stimulus presentation. Error shading is 1 SEM. n = 8 subjects. B: average BOLD activation during stimulus presentation across conditions. Pairwise comparisons show a significant decrease in V2 and V3 BOLD magnitude with eye closure for high temporal contrast stimuli. In LGN, BOLD magnitude with high temporal contrast stimuli with eyes open was marginally greater than low contrast [t(14)=1.79; P = 0.047] at a Bonferroni corrected P value cutoff of 0.0125. In V1 with eyes closed, BOLD magnitude during high temporal contrast stimuli was marginally greater than during low temporal contrast stimuli [t(14)=1.70; P = 0.055]. In V2, BOLD magnitude with high temporal contrast stimuli with eyes open was greater than low contrast [t(14)=2.51; P = 0.012] and high temporal contrast stimuli with eyes closed were suppressed compared with eyes open. In V3, BOLD magnitude during high temporal contrast stimuli with eyes closed was also suppressed compared with eyes open. The y-axis is BOLD signal averaged across 4–16 s poststimulus onset. Error bars are 1 SEM. All P values from pairwise comparison only survive multiple comparison correction at a P value less than 0.0125, using Bonferroni correction (0.05/n where n = 4 per ROI). *P < 0.0125. BOLD, blood oxygenation level-dependent; LGN, lateral geniculate nucleus; ROI, region-of-interest; V1, primary visual cortex; V2 and V3, extrastriate visual cortex.
Interestingly, while eye closure did not appear to have a major effect on the earliest stages of visual processing (LGN and V1), we observed a qualitatively distinct pattern within extrastriate cortices V2 and V3. When the eyes were closed, there was a drastic attenuation of stimulus-evoked responses, regardless of temporal contrast [main effects of eye closure: V2: F(1,31) = 6.45, P = 0.017; V3: F(1,31) = 5.79, P = 0.02; main effect of temporal contrast V2: F(1,31) = 2.91, P = 0.09; V3: F(1,31) = 0.54, P = 0.47]. Pairwise comparisons revealed a significant decrease in BOLD response to high temporal contrast stimuli with eye closure in V2 [t(14)=-3.13; P = 0.003) and V3 (t(14) = −3.09; P = 0.003]. Overall, these results indicate that visual processing appears to be qualitatively different with eyes closed compared with when eyes are open. The BOLD response in LGN and V1 was modulated by temporal contrast but was unaffected by eye closure, whereas eye closure strongly reduced responses in extrastriate cortices V2 and V3.
Along with the heterogeneity in patterns observed across striate and extrastriate regions, it is possible that there exists heterogeneity within each region. It has been reported that there is an eccentricity bias of the BOLD response in V1 and V2, when participants viewed center-surround stimuli with no local contrast (19). To test for an eccentricity bias and if eye closure impacts this bias, we separated voxels in V1–V3 by their eccentricity preference, based on pRF estimates (LGN was excluded from this analysis due to being underpowered for pRF analyses). We defined foveally-preferring voxels as those preferring between 0.01°–1.5° from fixation, parafoveal-preferring voxels were those between 1.5° and 4.0°, and peripheral-preferring voxels were between 4.0°–17.0°. As low temporal contrast trials elicited no significant activation across visuocortical regions, we did not test for an effect of eccentricity during low temporal contrast trials. We found that the effect of eccentricity was not significant in V1 [F(2,47) = 1.16, P = 0.333] (Fig. 3), nor in V2 [F(2,47) = 0.51, P = 0.606] nor V3 [F(2,47) = 0.30, P = 0.744]. No ROIs had any significant interaction between eye closure and eccentricity [V1: F(2,47) = 0.26, P = 0.772; V2: F(2,47) = 0.94, P = 0.397; V3: F(2,47) = 0.26, P = 0.768]. This suggests that across striate and extrastriate cortices there is no eccentricity bias in BOLD responses nor any difference with eye closure. Thus, the impact of high temporal contrast stimuli and eye closure on BOLD appear uniform within each visuocortical area.
Figure 3.
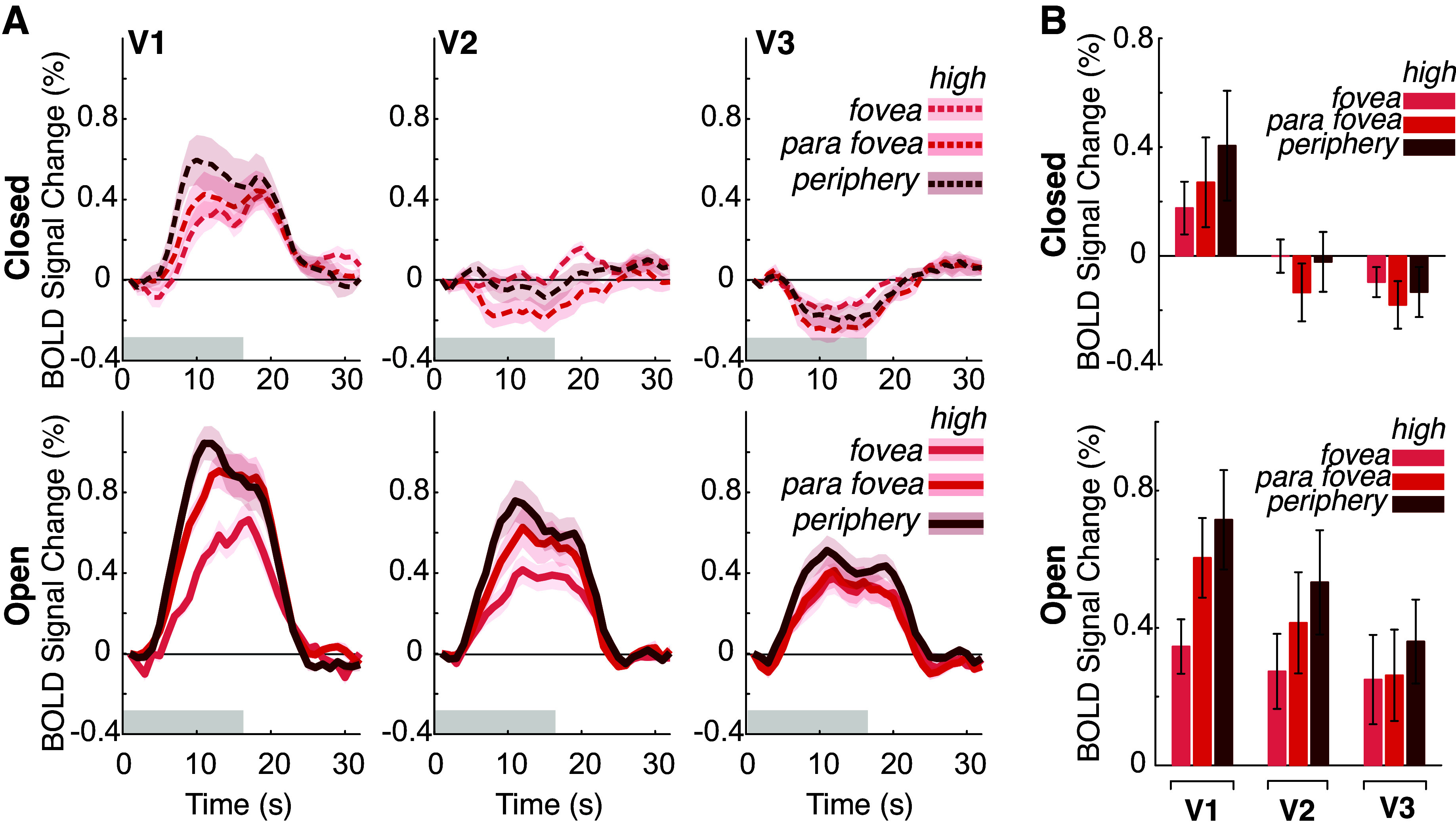
The effects of eye closure do not depend on eccentricity tuning. A: event-triggered average for BOLD response to luminance task across cortical ROI and eye condition separated by voxels tuned to different portions of the visual field. With eyes open and eyes closed, the BOLD responses to high-contrast stimuli are uniform across eccentricities for all cortical ROIs. Foveal voxels were tuned to between 0.01 dva and 1.5 dva. Parafoveal voxels were tuned to between 1.5 dva and 4.0 dva. Peripheral voxels were tuned to between 4.0 dva and 17 dva. B: average BOLD activation during stimulus presentation across conditions (top = eyes closed; bottom = eyes open), separated by eccentricity preference. There are no significant pairwise comparisons when comparing eccentricity responses within each ROI. The y-axis is BOLD signal averaged across 4–16 s poststimulus onset. Error bars and error shading are 1 SEM. BOLD, blood oxygenation level-dependent; ROI, region-of-interest; V1, primary visual cortex; V2 and V3, extrastriate visual cortex.
DISCUSSION
With subjective experience, it is clear that we can still perceive visual stimuli with closed eyes, but how distinct stages of the visual system supported this filtered visual experience was unknown. In this study, we found that eye closure produces a qualitatively distinct pattern of modulatory responses within the early visual system: closing one’s eyes selectively attenuated luminance processing in extrastriate cortex, but not in LGN or striate cortex.
In line with previous literature showing that early visual responses can still occur when the eyes are closed (5, 20), we demonstrated that with closed eyes, luminance-dependent responses remain present in the LGN and V1. However, we found substantial heterogeneity in activation across regions when eyes were closed. One hypothesis as to why we observed strongly attenuated BOLD with closed eyes in extrastriate cortex, but not the LGN nor striate cortex, is that top-down modulation of visuocortical responses is often stronger in extrastriate compared with striate cortex (21–23). It has been demonstrated that higher-order sensory regions, such as the frontal eye field (FEF), may account for the selective top-down modulation of extrastriate cortical responses (24). Resting-state fMRI studies that examined altered functional connectivity between eyes open and closed states found increased activation of the FEF during eyes closed relative to eyes open scans (7), lending further support to top-down modulation of extrastriate cortex during eyes closed states. Interestingly, one study which microstimulated the FEF of monkeys and measured visuocortical responses with fMRI found that FEF stimulation modulated extrastriate areas only in the presence of a visual stimulus, indicating that top-down modulation of the extrastriate cortices is dependent on bottom-up influence (25). Since our paradigm includes a visual stimulus, it is possible that eye closure in the presence of visual stimuli attenuates extrastriate cortical responses through both top-down and bottom-up mechanisms. In addition, the eyelid abolishes almost all structure and form-like information, which is necessary to elicit responses in extrastriate cortices that prefer higher-level feature selectivity, such as spatial contrast, shapes, and contours. However, eyelid closure still passes through luminance information, which is known to activate striate cortex (8). This preservation of luminance information, but attenuation of higher-level information, may explain the preservation of early visual pathway activation with weakened extrastriate activation.
Visuocortical responses have been shown to depend on luminance modulation, with responses increasing monotonically with luminance (8). In addition to luminance modulation, luminance response functions are strongly contrast dependent, with lower spatial contrast drastically decreasing visuocortical responses to luminance (8). Since the eyelid filters out much visual information, it is likely that spatial contrast no longer impacts visual responses and that luminance information dominates what might pass through the eyelid. In addition, the lower luminance retinal input with eye closure cannot fully explain our results since LGN and V1 showed no significant change in BOLD activation between open and closed eye conditions. Since the eyelid is characterized as a red-pass filter (3), it is possible that early visual pathways preferentially process this red visual content that extrastriate cortex is blind to; however, to our knowledge, no evidence of this exists. Although further work will be needed to better unpack luminance responses in the early visual system, our results suggest that luminance-based responses within early visual areas may not always necessitate the existence of spatial contrast to reveal themselves, as previously suggested.
There did not appear to be any significant effects of eccentricity on luminance responses, neither with eyes open nor eyes closed. Previous research found an eccentricity bias in BOLD responses in V1 and V2 to center-surround stimuli with no local contrast, most strongly at the edge between the center and surround of the stimuli (19). However, we may not have observed a similar effect simply because our visual stimulus did not include any spatial contrast, thereby precluding any edge effects from a center-surround stimulus. This suggests that the luminance-dependent effect of eccentricity might depend on where in the visual field an edge exists, which is consistent with another study examining luxotonic responses in the visual cortex using fMRI, which also did not find any effect of eccentricity on luxotonicity (8).
Our stimulus was designed to provide similar features of visual input across conditions, by presenting a diffuse light with no spatial contrast, but the input was nevertheless not identical when the eyes were closed due to the additional attenuation from the eyelid. Future experiments could test a stimulus that mimics the reduced retinal input of the eyelid. This would involve measuring the spectral transmittance of each participant’s eyelid and adjusting the high temporal contrast visual stimulus to account for the attenuated transmittance. Such a stimulus would necessarily abolish all structure and form uniformly across the visual scene, which under standard models of center-surround neuronal receptive field organization would predict no net change in visuocortical responses due to equivalent stimulation across excitatory and inhibitory components of the visual cortex (26, 27). However, recent findings of luminance modulation within the visual cortex suggest that luminance information alone can drive the visual system (8). Thus, a stimulus mimicking the eyelid preserves luminance information and would be expected to still activate the visual system, in accordance with our eyes closed condition in which high-intensity stimuli still activate LGN and V1. If the visual thalamus and visuocortical responses between a stimulus mimicking the eyelid with open eyes and the high-intensity stimulus in our eyes closed condition did not align, this would suggest that the physical properties of the eyelid are not sufficient to explain our results.
An alternative explanation of our results is that modulation of visual processing during eye closure may be dependent on brain state, not just the physical barrier of the eyelid. Eye closure likely induces a change in the overall brain state that alters both the processing of visual information and large-scale functional network processing. Eye closure decreases activity in attentional systems in the occipital and parietal lobes and increases functional coupling between sensory thalamus and somatosensory regions (5–7). Even during short eye blinks, there is evidence of increased activity in the default mode network (DMN) and decreased activity in the dorsal attention network, suggesting attentional disengagement even with short eye closures (28). During longer eye closures, as examined in this study, we might expect prolonged or even greater magnitude DMN activation, supporting this idea of attentional disengagement with closed eyes. These differences in spontaneous brain activity across sensory and attentional systems point to altered brain states with eye closure. Exteroceptive and interoceptive mental state hypotheses have been formulated where an exteroceptive mental state is characterized by increased attention and sensory processing of the external environment with eyes open (5). On the other hand, an interoceptive mental state is characterized by internally directed cognition and reduced sensory processing with eyes closed. Many brain states require prolonged periods of eye closure, such as sleep and meditation, that involve reduced sensory awareness of external stimuli and enhanced internally directed attention. Thus, eye closure may modulate visual processing through attentional or brain-state-dependent mechanisms.
DATA AVAILABILITY
Source data for this study are openly available at: https://doi.org/10.18112/openneuro.ds005194.v1.1.0.
GRANTS
This work was supported by National Institutes of Health R01 EY028163 (to S.L.), Sloan Fellowship (to L.D.L.), McKnight Scholar Award (to L.D.L.), Pew Biomedical Scholar Award (to L.D.L.), and NIH U19-NS128613 (to L.D.L.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.G.C., M.K., L.D.L., and S.L. conceived and designed research; N.G.C. and M.K. performed experiments; N.G.C. analyzed data; N.G.C., M.K., L.D.L., and S.L. interpreted results of experiments; N.G.C. prepared figures; N.G.C. drafted manuscript; N.G.C., M.K., L.D.L., and S.L. edited and revised manuscript; N.G.C., M.K., L.D.L., and S.L. approved final version of manuscript.
ACKNOWLEDGMENTS
This research was carried out at the Boston University Cognitive Neuroimaging Center. This work involved the use of instrumentation supported by the NSF Major Research Instrumentation grant BCS-1625552. We acknowledge the University of Minnesota Center for Magnetic Resonance Research for use of the multiband-EPI pulse sequences. Data were analyzed on a high-performance computing cluster supported by the ONR grant N00014-17-1-2304. We thank Shruthi Chakrapani and Stephanie McMains for assistance with data collection, and members of the S.L. laboratory and L.D.L. laboratory for helpful feedback on the manuscript.
REFERENCES
- 1. Blume C, Garbazza C, Spitschan M. Effects of light on human circadian rhythms, sleep and mood. Somnologie 23: 147–156, 2019. doi: 10.1007/s11818-019-00215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ohayon MM, Milesi C. Artificial outdoor nighttime lights associate with altered sleep behavior in the American general population. Sleep 39: 1311–1320, 2016. doi: 10.5665/sleep.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ando K, Kripke DF. Light attenuation by the human eyelid. Biol Psychiatry 39: 22–25, 1996. doi: 10.1016/0006-3223(95)00109-3. [DOI] [PubMed] [Google Scholar]
- 4. Bierman A, Figueiro MG, Rea MS. Measuring and predicting eyelid spectral transmittance. J Biomed Opt 16: 067011, 2011. doi: 10.1117/1.3593151. [DOI] [PubMed] [Google Scholar]
- 5. Marx E, Deutschländer A, Stepha T, Dieterich M, Wiesmann M, Brandt T. Eyes open and eyes closed as rest conditions: Impact on brain activation patterns. NeuroImage 21: 1818–1824, 2003. doi: 10.1016/j.neuroimage.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 6. Wei J, Chen T, Li C, Liu G, Qiu J, Wei D. Eyes-open and eyes-closed resting states with opposite brain activity in sensorimotor and occipital regions: Multidimensional evidences from machine learning perspective. Front Hum Neurosci 12: 422, 2018., doi: 10.3389/fnhum.2018.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weng Y, Liu X, Hu H, Huang H, Zheng S, Chen Q, Song J, Cao B, Wang J, Wang S, Huang R. Open eyes and closed eyes elicit different temporal properties of brain functional networks. NeuroImage 222: 117230, 2020. doi: 10.1016/j.neuroimage.2020.117230. [DOI] [PubMed] [Google Scholar]
- 8. Vinke LN, Ling S. Luminance potentiates human visuocortical responses. J Neurophysiol 123: 473–483, 2020. doi: 10.1152/jn.00589.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brainard DH. The psychophysics toolbox. Spat Vis 10: 433–436, 1997. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- 10. Dumoulin SO, Wandell BA. Population receptive field estimates in human visual cortex. NeuroImage 39: 647–660, 2008. doi: 10.1016/j.neuroimage.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kay KN, Winawer J, Mezer A, Wandell BA. Compressive spatial summation in human visual cortex. J Neurophysiol 110: 481–494, 2013. doi: 10.1152/jn.00105.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujita N, Tanaka H, Takanashi M, Hirabuki N, Abe K, Yoshimura H, Nakamura H. Lateral geniculate nucleus: anatomic and functional identification by use of MR imaging. Am J Neuroradiol 22: 1719–1726, 2001. [PMC free article] [PubMed] [Google Scholar]
- 13. Ling S, Pratte MS, Tong F. Attention alters orientation processing in the human lateral geniculate nucleus. Nat Neurosci 18: 496–498, 2015. doi: 10.1038/nn.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fischl B. FreeSurfer. NeuroImage 62: 774–781, 2012. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. NeuroImage 20: 870–888, 2003. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- 16. Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage 48: 63–72, 2009. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. NeuroImage 53: 1181–1196, 2011. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106: 1125–1165, 2011. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cornelissen FW, Wade AR, Vladusich T, Dougherty RF, Wandell BA. No functional magnetic resonance imaging evidence for brightness and color filling-in in early human visual cortex. J Neurosci 26: 3634–3641, 2006. doi: 10.1523/JNEUROSCI.4382-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharon O, Nir Y. Attenuated fast steady-state visual evoked potentials during human sleep. Cerebral Cortex 28: 1297–1311, 2018. doi: 10.1093/cercor/bhx043. [DOI] [PubMed] [Google Scholar]
- 21. Haenny PE, Schiller PH. State dependent activity in monkey visual cortex. Exp Brain Res 69: 225–244, 1988. doi: 10.1007/BF00247569. [DOI] [PubMed] [Google Scholar]
- 22. Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science 229: 782–784, 1985. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 23. Shulman G, Corbetta M, Buckner RL, Raichle ME, Fiez JA, Miezin FM, Petersen SE. Top-down modulation of early sensory cortex. Cerebral Cortex 7: 193–206, 1997. doi: 10.1093/cercor/7.3.193. [DOI] [PubMed] [Google Scholar]
- 24. Veniero D, Gross J, Morand S, Duecker F, Sack AT, Thut G. Top-down control of visual cortex by the frontal eye fields through oscillatory realignment. Nat Commun 12: 1757, 2021. doi: 10.1038/s41467-021-21979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ekstrom LB, Roelfsema PR, Arsenault JT, Bonmassar G, Vanduffel W. Bottom-up dependent gating of frontal signals in early visual cortex. Science 321: 414–417, 2008. doi: 10.1126/science.1153276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Movshon JA, Thompson ID, Tolhurst DJ. Spatial summation in the receptive field of simple cells in the cat’s striate cortex. J Physiol 283: 53–77, 1798. doi: 10.1113/jphysiol.1978.sp012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ringach DL. Mapping receptive fields in primary visual cortex. J Physiol 558: 717–728, 2004. doi: 10.1113/jphysiol.2004.065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakano T, Kato M, Morito Y, Itoi S, Kitazawa S. Blink-related momentary activation of the default mode network while viewing videos. Proc Natl Acad Sci USA 110: 702–706, 2013. doi: 10.1073/pnas.1214804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Source data for this study are openly available at: https://doi.org/10.18112/openneuro.ds005194.v1.1.0.


