Highlights
-
•
Females improved executive function after three resistance exercises unlike males.
-
•
Internal carotid artery blood flow increased equivalently for the males and females.
-
•
No condition differences for increases in internal carotid artery blood flow.
-
•
Females exhibited an attenuated increase in blood pressure compared to the males.
Keywords: Executive function, Cognitive flexibility, Blood flow restriction, Internal carotid artery, Cerebral blood Flow, Sex differences
Abstract
The aim was to examine the effects of modalities of acute resistance exercise (RE) on cognition and hemodynamics including internal carotid artery (ICA) blood flow (BF). Twenty adults completed familiarization and experimental visits. One-repetition maximum (1RM) for bilateral leg extension was quantified, and baseline executive functioning was determined from three run-in visits. Subsequent visits included three randomized, volume-equated, acute exercise bouts of 30 %1RM+blood flow restriction (BFR), 30 %1RM, and 70 %1RM. Both 30 %1RM trials completed four sets of exercise (1 × 30, 3 × 15), and the 70 %1RM condition completed four sets of 8 repetitions. BFR was induced with 40 % of the pressure to occlude the femoral arteries. 11 min following each exercise, participants completed the Stroop and Shifting Attention Tests. Baseline and post-exercise values were used to calculate change scores. The resulting mean change scores were evaluated with mixed factorial ANOVAs. A p≤0.05 was considered significant. All measured outcome variables increased in response to exercise. The ANOVAs for cognitive scores indicated no significant (p>0.05) interactions. For cognitive flexibility and executive function index, there were main effects of Sex. Change scores of the females were significantly greater than the males for cognitive flexibility (7.6 ± 5.9 vs. -2.6 ± 8.4 au; p=0.007) and executive function index (7.4 ± 4.6 vs. -2.5 ± 6.5 au; p=0.001). For ICA BF, there was no significant interaction or any main effect. The females exhibited a smaller exercise-induced increase in blood pressure compared to the males (17.7 ± 5.9 vs. 11.0 ± 4.1 mmHg; p=0.010). Each RE modality yielded acute improvements in cognition, but only for females. There were no cognitive improvements related to BFR such that each RE bout yielded similar results.
1. Introduction
Non-pharmacological approaches to prevent and/or delay the onset of Alzheimer's Disease and related dementias continue to garner substantial interest in response to the current lack of available disease-modifying drugs [[1], [2], [3]]. Lifestyle-based approaches, including various forms of exercise, can reduce known modifiable risk factors (e.g., vascular contributions to cognitive impairment and dementia) associated with cognitive decline resulting from Alzheimer's Disease and related dementias [[4], [5], [6], [7], [8], [9], [10], [11], [12]]. A majority of the previous research examining the benefits of lifestyle-based preventive interventions has primarily focused on aerobic exercises [5,6,13,14] despite substantiated evidence of cognitive benefits derived from resistance exercise (RE) [[15], [16], [17], [18]]. Further, most studies aimed at slowing cognitive decline primarily investigate older adults, despite younger individuals possibly offering a more attractive model due to a lack of age-related damage to organs such as the brain. Moffitt et al. [19] explicitly stated that gerontology topics of health-span extension should investigate young people. Blood flow restriction (BFR) with RE (BFR+RE), which has recently been hypothesized to promote cognitive enhancement to a greater extent than traditional RE, is a rational candidate for the investigation of a lifestyle-based approach for health-span improvement in young adults [[20], [21], [22]]. Specifically, BFR+RE utilizes commercially available cuffs applied to the uppermost portion of a limb to fully occlude venous outflow while only partially limiting arterial inflow [23]. An additional hallmark of BFR+RE includes using low loads as opposed to more commonly prescribed high loads corresponding to ≥70 % of one-repetition maximums (1RM) [23,24]. Sardeli et al. [22] reported that in 24 older adults, there was a greater acute effect of low load-BFR+RE on Stroop Test performance compared to a high load-non-BFR condition. Notably, these investigators utilized a bilateral lower body exercise (leg press), which was likely chosen due to the well-accepted principle that the amount of muscle mass recruited increases neuronal activity and hemodynamics in a dose response manner [[25], [26], [27], [28]]. Thus, it is possible that the observed acute cognitive enhancement was elicited by superior cerebral blood flow (CBF) and, thus, greater cerebral oxygenation facilitated by lower body (i.e., sufficient muscle mass recruited), low load-BFR+RE [20,29]. However, no previous study has directly tested whether BFR+RE influences CBF to a different extent than other volume-matched conditions. One appropriate technique to address this gap includes assessing internal carotid artery (ICA) blood flow (BF) pre- and post-RE. For instance, during exercise, increases in ICA BF match increases in cerebral metabolism [30], and during exercise absent of hyperventilation, middle cerebral artery and ICA BF similarly increased [31]. Further, an exercise-induced increase in CBF has been identified as a potential factor contributing to acute cognitive enhancement following exercise bouts [32,33], yet conflicting reports exist [30]. Examining the potential link between exercise-induced increases in ICA BF and cognition remains especially important considering the lack of effective pharmaceutical treatment as well as the currently unspecified best exercise prescription for preventing cognitive decline.
In addition to determining the potentially different effects of BFR+RE on CBF and cognition compared to traditional RE, examining these responses in a sex-specific manner is necessary. Females remain largely understudied in several lines of biomedical research, yet there are known sex differences in exercise-induced changes to cognition [8,14,[34], [35], [36], [37]] as well as in the relations between fitness and cerebral hemodynamics [38]. The sex-specific cognitive changes have previously been hypothesized to be attributed to differences in sex steroid hormone profiles as well as the expression and influence of ‘exerkines’ like brain derived neurotrophic factor (BDNF) [34]. Further, in terms of the sex differences in cerebral hemodynamics, the exercise pressor reflex may partially contribute to these reported differences considering females typically exhibit an attenuated response [[39], [40], [41], [42]]. Specifically, the exercise pressor reflex increases cardiovascular factors to meet metabolic demands while also maintaining perfusion to organs such as the brain [41,43,44]. This reflex is provoked by mechanical and metabolic stimuli that act on group III and IV skeletal muscle free nerve endings during physical activity, including bouts absent of substantial acidosis and lactate accumulation [28,45] as well as without the input from the cardiorespiratory center in the brainstem [43,44]. As mentioned above, females generally display lower increases in blood pressure compared to males during matched exercise, which may lead to differences in potential hemodynamic-linked mechanisms contributing to changes in acute cognition. However, to date, sex-specific acute changes in ICA BF and cognition have not been examined in response to BFR+RE, especially since females are underrepresented in BFR+RE related research as well [23,46]. Taken together, this further highlights the present need to probe potential sex differences.
Therefore, the primary aim of the current study was to examine the potential sex-specific acute effects of three distinct volume-matched RE modalities on ICA BF and cognition. The three modalities included low load-BFR+RE, low load-non-BFR+RE, and high load-non-BFR+RE conditions in healthy, young males and females. Based on previous studies [20,22,29,34,47], it was hypothesized that ICA BF and cognition would increase in response to all three RE modalities; however, females would show greater enhancements. Additionally, it was hypothesized that low load-BFR+RE would elicit the greatest cognitive improvements. Testing these hypotheses was critically important as highlighted by previous work that demonstrated acute effects were associated with chronic, training-induced effects on executive functioning [48]. That is, acute responses can provide insight into potential chronic adaptations resulting from longer duration training studies.
2. Methods
2.1. Ethics approval
This study was completed according to the ethics standards established by the Declaration of Helsinki 2013 and was approved by the local Institutional Review Board for Human Subjects at the University of South Alabama (IRB no. 23-131/2042716-2). We did not register this study in a publicly available database. Prior to the initiation of any experimental procedure, all participants gave signed, written informed consent. No data related to this project has been previously published or submitted in any form.
2.2. Experimental design
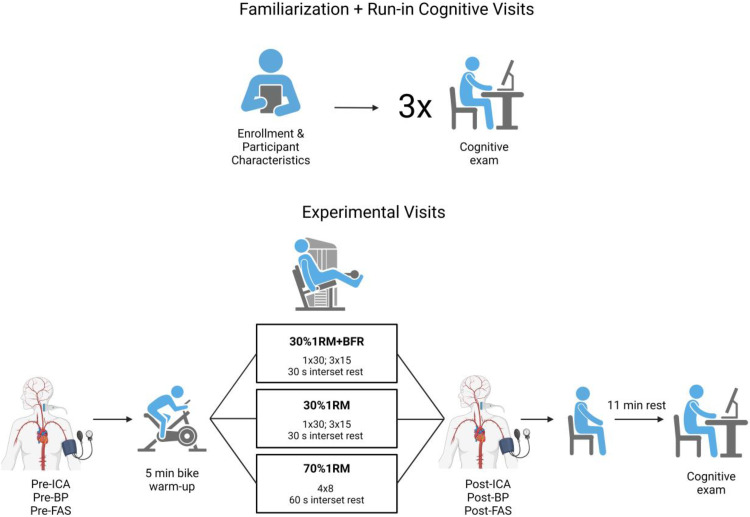
To address the purpose of the current study, a randomized, counterbalanced experimental design was used (Fig. 1). All consenting participants visited the Integrative Laboratory of Exercise and Applied Physiology (iLEAP) for six visits: three run-in baseline cognitive assessments, including familiarization procedures, and three experimental visits. These visits were largely scheduled on the basis of participant convenience, but strong attempts were made to schedule all visits before noon. Familiarization procedures included determining the minimum arterial occlusion pressure (AOP) necessary for each femoral artery to set individualized, relative BFR cuff pressures. Additionally, familiarization consisted of measuring 1RM values for bilateral leg extension. Once all three baseline cognitive visits were completed, the experimental visits were performed in a randomized order. These consisted of three different, acute RE bouts that were volume-equated to negate a potential nuisance effect on cognition due to discrepancies in exercise volume (i.e., repetitions x load). The three conditions included low load and high load non-BFR bouts as well as a low load BFR bout. The main outcomes of cognitive scores and ICA values were recorded pre- and post-exercise to determine the exercise-induced magnitude of change. Arousal levels were also recorded. All procedures and tests were performed in the temperature- (21–22 °C) and ambient light-controlled iLEAP.
Fig. 1.
Experimental design of the investigation depicting the familiarization visit and three run-in cognitive exams. Additionally, pre- and post-internal carotid artery (ICA), blood pressure (BP), and Felt arousal scale (FAS) measures are presented, which occurred during three, randomized conditions performed on separate days: 30 % 1-repetition maximal (1RM) plus blood flow restriction (BFR) at 40 % of minimum arterial occlusion pressure, 30 % 1RM non-BFR, and 70 % 1RM non-BFR.
2.3. Human participants
A total of 23 participants were recruited for this investigation. However, due to repetitive scheduling conflicts (n=1), a previously unknown medical condition (n=1), and signal interruption (n=1), three participants did not complete all experimental visits. Thus, 20 self-reported recreationally active [49], young (19–35 yr) adults (11 males, nine females) with no cardiovascular, metabolic, neuromuscular, or renal diseases were used for subsequent data analysis (Table 1). Sex was self-reported and corresponded to sex assigned at birth. No attempt was made to control for the menstrual cycle. Based on previous findings and the purpose of the current study, it was our interpretation that the likely variation in menstrual cycle phases did not substantially affect the results of the present study, especially considering the randomization of the exercise bouts [[50], [51], [52], [53]]. Participants were encouraged to maintain their normal dietary habits and continue their personal exercise programs without significant alteration. However, the participants were asked to abstain from exercise for at least 24 hours prior to each visit. Participants completed all three experimental visits in 12 ± 5 days. For descriptive purposes, body composition was assessed using a Fit3D body scan (FIT3DⓇ ProScanner™, Redwood City, CA) [54].
Table 1.
Participant characteristics.
| Male (n=11) | Female (n=9) | 95 % CI Mean Difference; p-value | |
|---|---|---|---|
| Age (yr) | 22 ± 5 | 21 ± 2 | -4.6 to 2.5; p=0.551 |
| Height (cm) | 177.8 ± 9.3 | 164.6 ± 4.4 | -20.3 to -6.1; p=0.001 |
| Weight (kg) | 84.9 ± 11.8 | 63.1 ± 7.5 | -31.4 to -12.3; p<0.001 |
| Body Mass Index (kg∙m2) | 27.0 ± 4.3 | 23.2 ± 2.0 | -7.1 to -0.462; p=0.028 |
| Lean Mass (kg) | 67.2 ± 7.7 | 47.5 ± 5.0 | -25.9 to -13.4; p<0.001 |
| Fat Mass (kg) | 17.9 ± 5.4 | 16.4 ± 2.2 | -5.6 to 2.6; p=0.450 |
| Body Fat Percentage (%) | 20.8 ± 4.3 | 26.4 ± 1.8 | 2.5 to 8.9; p=0.002 |
| Systolic Blood Pressure (mmHg) | 128 ± 8 | 115 ± 6 | -19.3 to -6.1; p<0.001 |
| Diastolic Blood Pressure (mmHg) | 74 ± 5 | 67 ± 8 | -13.1 to -0.431; p=0.038 |
| Mean Arterial Pressure (mmHg) | 97 ± 8 | 83 ± 6 | -20.8 to -7.9; p<0.001 |
| Resting Heart Rate (BPM) | 67 ± 10 | 69 ± 10 | -7.1 to 11.7; p=0.614 |
| Right Occlusion Pressure (mmHg) | 151 ± 20 | 132 ± 8 | -33.3 to -3.6; p=0.018 |
| Left Occlusion Pressure (mmHg) | 155 ± 20 | 132 ± 5 | -37.4 to -8.1; p=0.004 |
| Bilateral Leg Extension 1RM (lbs) | 245 ± 39 | 141 ± 25 | -136.1 to 72.7; p<0.001 |
Note: Mean differences calculated Females – Males. Bolded p-values demarcate a significant (p≤0.05) mean difference between the males and females. Abbreviations: Confidence interval (CI); Beats per minute (BPM); One-repetition maximum (1RM)
2.4. Power analysis
Based on previous investigations, a power analysis (G*Power v.3.1.9.7, Düsseldorf, Germany) was conducted to determine a recommended sample size range for detecting (α = 0.05 and β = 0.80) exercise-induced changes in cognition from pre- to post-exercise. An effect size of 0.86 was determined from Dora et al. [55] who reported an improvement in reaction time from baseline to 10-min post-RE. This yielded a recommended total sample size of 13. Anders et al. [18] studied the effects of acute high load-RE on cognitive function as determined by the Automated Neuropsychological Assessment Metrics. This investigation suggested there was an enhancement (effect size = 0.81) for mathematical processing from familiarization to post-exercise, which yielded a recommendation of n=14. Taken together, the current sample was likely sufficient to detect exercise-induced changes in cognition.
2.5. Familiarization procedures
During the first visit to the laboratory (i.e., cognitive run-in #1), the participants reviewed and completed all documentation associated with the study. Height, weight, and hand dominance (based on self-reported throwing preference) were also recorded during the first visit. The first task included asking the participants to lay supine on a padded treatment table and rest quietly. Resting blood pressure was first recorded and then the minimum AOP of each femoral artery was quantified via commonly reported accepted procedures [23,24,56]. The circumference of each thigh (at the midpoint) was measured, which was then followed by wrapping a commercially-available cuff (SC12LTM 12 × 124 cm; Hokanson Inc., Belleview, WA, USA) around the uppermost portion of either the right or left thigh. This cuff was systematically inflated while simultaneously viewing arterial BF within the popliteal artery via Doppler ultrasound (Logiq E R7-Next Gen, GE Healthcare, USA). Specifically, the cuff was inflated to 50 mmHg to ensure proper cuff attachment and ultrasound placement. The cuff was then rapidly deflated and then re-inflated in 50 mmHg increments until complete occlusion occurred (i.e., absence of flow through popliteal artery due to upstream occlusion). Once this pressure was identified, the cuff remained inflated, but the cuff pressure was gradually lowered until popliteal arterial flow was again observed. We then increased one mmHg∙s-1 until flow again disappeared. This pressure was defined as the minimal AOP. These procedures were repeated on the contralateral limb, and if the pressures differed by >10 %, both limbs were reassessed.
Following the AOP procedures, the participants were asked to move to the bilateral leg extension machine (VR2 Leg extension with part no. 4850, Cybex International Owatonna, MN). The seat height was adjusted such that the lateral epicondyle of the femur aligned with the axis of rotation, and while seated in this position, the participants were oriented to the Felt Arousal Scale (FAS) [57]. Next, ultrasonography of the ICA was demonstrated and performed on the participants for familiarization purposes. Once comfortable with the FAS and ultrasound techniques, the standardized bilateral leg extension warmup was initiated. This included a 5 min warmup on a stationary bike (Ergomedic 828 E, Monark, Verberg, Sweden) and 2–3 sets of 3–12 repetitions of submaximal leg extensions corresponding to estimated loads of 40–80 % 1RM. Based on subjective feedback, the load was progressively increased until the participant could no longer complete a leg extension through the full range of motion. The heaviest load lifted across the entire range of motion was defined as the 1RM. Strong verbal encouragement was provided during each 1RM attempt. Participants were also instructed to grasp handles near the seat during all contractions. Their upper body was not restricted, but the participants were instructed to keep their backs against the backrest and to prevent their hips from raising. Lastly, following the identification of the 1RM, participants were instructed on the procedures associated with the cognitive assessment and then began the test.
2.5.1. Cognitive testing
All cognitive testing took place in a quiet room. Two executive functioning subtests from the CNS Vital Signs platform were utilized in this study: the Stroop Test and the Shifting Attention Test (SAT) [58]. First, the participants completed three phases of the Stroop test which included assessing simple reaction time, congruent reaction time and accuracy, and incongruent reaction time and accuracy. Participants then completed the SAT, which instructed them to follow changing directions of matching colors or shapes. Both subtests measure set-shifting ability and inhibitory control. After completing these two tests, CNS Vital Signs domains of reaction time, cognitive flexibility, and executive function index were calculated. Reaction time was calculated from the sum of correct Stroop Test Complex Reaction Time and Stroop Reaction Time responses divided by 2 (i.e., Reaction time = ∑ (Correct Stroop Test Complex and Stroop Reaction Time) ÷ 2). Cognitive flexibility was reported as total SAT correct responses subtracted by SAT errors and Stroop commission errors (i.e., Cognitive flexibility = SAT correct – (SAT errors + Stroop commission errors)). Lastly, the executive function index was determined by subtracting SAT errors from total correct SAT responses (i.e., Executive function = SAT correct – SAT errors). All of these calculations were performed using the CNS Vital Signs software. That is, the formulas for these clinical domain scores are inherent to the CNS Vital Signs platform. The scores used for analysis were standardized to data within the CNS Vital Signs platform. These standardized scores were used for all subsequent analyses [12,58].
2.5.2. Felt arousal scale (FAS)
The FAS is a 6-point scale that measures perceived arousal from low arousal (1 point) to high arousal (6 points). The participants were anchored to the scale by describing the lowest value as corresponding to feelings associated with lethargy and unstimulated, whereas the highest value was loaded with terms such as feeling excited and being very alert [57]. Evaluating FAS strengthened the appropriateness of comparing changes in cognitive scores such that some context was provided by quantifying potential differences in the arousal level of each individual. Much of the previous research examining relations between exercise and cognition was based on arousal theories [59].
2.5.3. Ultrasonography - internal carotid artery (ICA)
The left ICA was evaluated for all participants due to the setup and design of the leg extension machine. The ultrasound probe (12L-Rs; 5–13 MHz; 38.4 mm field-of-view) tracked the common carotid artery until the bifurcation into the ICA and external carotid artery. The most inferior portion (i.e., 1–2 cm above bifurcation) of the ICA demonstrating clear vessel walls was insonated at 60°. Blood velocity was assessed at this anatomical location by determining the maximum frequency (TAMAX), time-averaged peak velocity, across three complete cardiac cycles. The ICA was confirmed via the Doppler-derived pulse waveforms. Vessel diameter was collected from a still photo using the straight-line function. To calculate ICA BF, the following equation was used: . Great care was given to not compress the artery, and to enhance acoustic coupling, a water-soluble gel was generously applied to the probe and skin.
2.6. Experimental visit procedures
Upon arrival, the participants were directed to sit quietly in the leg extension machine, and the lever arm was adjusted to previously determined settings. After five minutes of quiet sitting, arousal was assessed with the FAS, BP, and HR were measured with a standard patient monitor (Datascope Passport 2 Patient Monitor, Montvale, NJ), and pre-exercise ICA BF was quantified. Participants then completed a 5 min submaximal stationary cycle ergometry bout. Once warm-up was completed, the participants completed one of three randomized, counter-balanced, volume-matched RE bouts. These included low load leg extensions at 30 % 1RM with and without BFR for a total of 75 repetitions (1 × 30, 3 × 15, 30 s inter-set rest) as well as a non-BFR high load bout at 70 % 1RM for a total of 32 repetitions (4 × 8, 1 min inter-set rest). During the low load-BFR+RE condition, independently controlled cuffs were wrapped around the uppermost portion of each thigh and inflated to a pressure corresponding to 40 % of the previously determined AOP [23]. The cuffs remained inflated between sets and were only deflated once every measure was collected post-exercise (e.g., FAS and ICA BF). Post-exercise measures also included BP, which was reported as mean arterial pressure (MAP). Immediately after the last repetition of each condition, a stopwatch was started. Participants started the post-exercise cognitive assessment precisely 11 min after the last repetition to capture the largest effects of exercise on cognitive performance [60,61]. After completing this, the participants were invited back to the laboratory on separate days to complete the remaining conditions.
2.7. Data and Statistical Analysis
Difference scores were calculated to assess the magnitude of change that occurred in response to each specific RE modality for each outcome variable (FAS, MAP, Reaction Time, Cognitive Flexibility, Executive Function Index, and ICA BF). Specifically, individual participant difference scores were calculated as post-exercise – baseline. The cognitive baseline values were defined as the maximum value observed across the three run-in trials for each participant. To highlight the circumvented potential practice effect, 1-way repeated measures ANOVAs were conducted for each cognitive domain across the three run-in trials. The non-cognitive baseline values (FAS, MAP, ICA BF) were collected immediately prior to exercise. Separate, 2 × 3 (Sex × Exercise Modality) mixed factorial ANOVAs were used to determine potential mean differences in the magnitude of change. In the absence of a significant interaction, the main effects were evaluated with Bonferroni-corrected pairwise comparisons. Partial eta squared () was used as a measure of effect for each ANOVA model. The Greenhaus-Geisser correction was applied when sphericity was not met (assessed with Maulchy's Test of Sphericity). Independent t-tests were used to examine potential mean differences in participant characteristics between the males and females (Table 1). 95 % confidence intervals (CI) of the mean differences were reported in addition to p-values. All calculations and statistical analyses were conducted utilizing Statistical Package for the Social Sciences software (version 26.0. IBM Inc. Chicago, Ill, USA) and GraphPad Prism (version 8.3.4. GraphPad Software, San Diego, CA, USA). A p-value ≤ 0.05 was considered statistically significant. All data were reported as mean ± SD.
3. Results
3.1. Determination of practice effect
For reaction time, there was a significant (p = 0.023, = 0.342) practice effect such that the first trial exhibited the lowest mean (104.7 ± 15.2), but there was no difference (p = 0.741) between the second (111.4 ± 11.2) and third trials (111.7 ± 10.3). For cognitive flexibility, there was a significant (p < 0.001, = 0.526) trial effect such that scores significantly and consecutively improved across the three trials. Specifically, cognitive flexibility was significantly lower during the first trial (102.8 ± 15.6) compared to the second (112.1 ± 12.9; p = 0.003) and third (118.1 ± 11.8; p < 0.001) trials, and the third trial was significantly (p = 0.003) greater than the second trial. Similarly, for executive function index, there was a significant (p < 0.001, = 0.540) trial effect across the three run-in cognitive assessment trials. Executive function index was significantly lower during the first trial (103.8 ± 14.8) compared to the second (113.0 ± 12.0; p = 0.003) and third (119.3 ± 10.8; p < 0.001) trials, and the third trial was significantly (p = 0.010) greater than the second trial. These mixed findings led to the selection of the best performance of the first three trials being used for the individual's baseline measure for the calculation of change scores.
3.2. Exercise-induced change in cognition
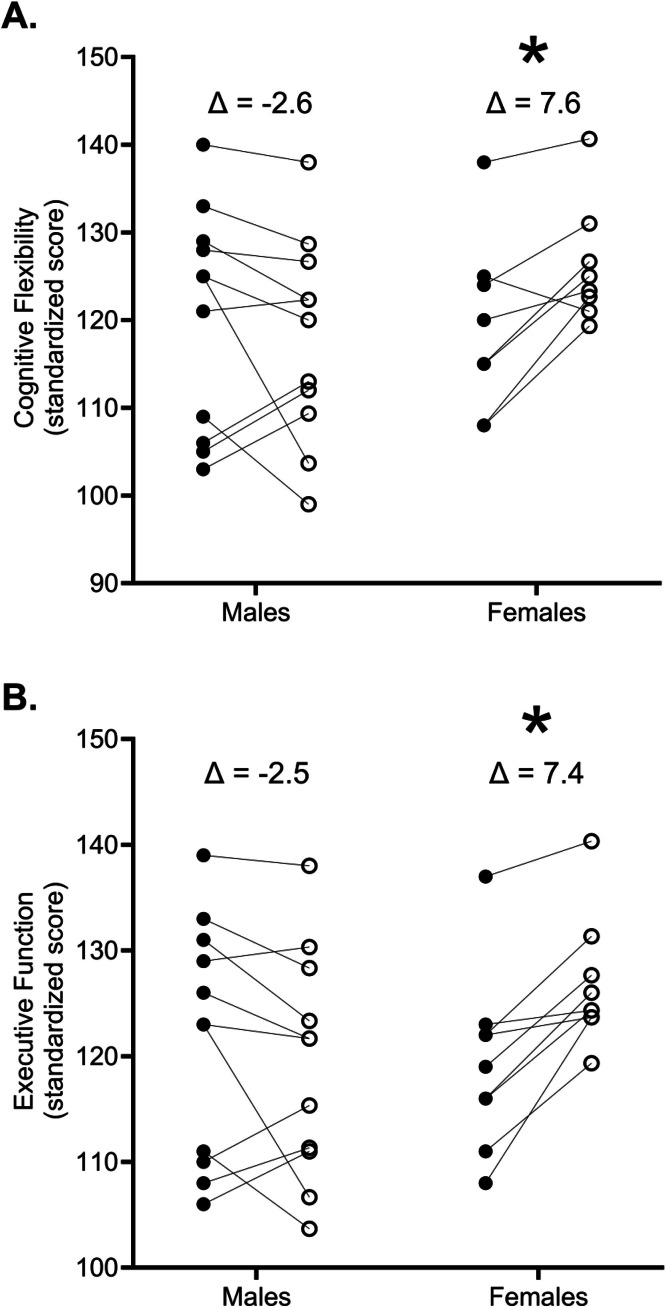
For reaction time, there was not a significant interaction (p = 0.745, = 0.016) nor a main effect for Exercise Modality (p = 0.839, = 0.010) or Sex (p = 0.528, = 0.022). There was also no significant interaction for cognitive flexibility (p = 0.651, = 0.024), but there was a significant main effect for Sex (p = 0.007, = 0.343). The follow-up Bonferroni-corrected pairwise comparisons indicated that the females (7.6 ± 5.9) exhibited a significantly (p = 0.007) greater improvement from baseline than the males (-2.6 ± 8.4) independent of modality (Fig. 2). Similarly, for executive function index, there was not a significant interaction (p = 0.625, = 0.026), but there was a significant main effect for Sex (p = 0.001, = 0.453). The follow-up Bonferroni-corrected pairwise comparisons indicated that the females (7.4 ± 4.6) exhibited a significantly (p = 0.001) greater improvement than the males (-2.5 ± 6.5).
Fig. 2.
Panel A: Individual changes (collapsed across resistance exercise modality) from pre-exercise (●) to post-exercise (ס) in cognitive flexibility. There was a significant (*p = 0.007) mean difference (Δ) between the males (-2.6 ± 8.4) and females (7.6 ± 5.9). Panel B: Individual changes (collapsed across resistance exercise modality) from pre- to post-exercise in executive function index. There was a significant (p = 0.001) mean difference between the males (-2.5 ± 6.5) and females (7.4 ± 4.6). The data depicted in each panel are scores that were standardized to data available within the CNS vital signs platform.
3.3. Exercise-induced change in arousal, MAP, and ICA BF
There was not a significant interaction (p = 0.745, = 0.016) nor any main effect (Modality: p = 0.135, = 0.105; Sex: p = 0.738, = 0.006) for the FAS-derived arousal change scores. For MAP, there was not a significant (p = 0.635, = 0.025) interaction, but there was a significant main effect for Sex (p = 0.010, = 0.315) (Fig. 3). The Bonferroni-corrected pairwise comparisons indicated that the males (17.7 ± 5.9 mmHg) exhibited a significantly (p = 0.010) greater increase in MAP than the females (11.0 ± 4.1 mmHg). For ICA BF, as shown in Fig. 4, there was not a significant interaction (p = 0.529, = 0.035) nor any main effect (Modality: p = 0.935, = 0.004; Sex: p = 0.105, = 0.139).
Fig. 3.
Individual changes (collapsed across resistance exercise modality) from pre-exercise (●) to post-exercise (ס) in mean arterial pressure. There was a significant (*p = 0.010) mean difference (Δ) between the males (17.7 ± 5.9 mmHg) and females (11.0 ± 4.1 mmHg) such that the females increased to a lesser extent.
Fig. 4.
The low-load (LL) blood flow restriction (BFR) (Panel A), LL-non-BFR (Panel B), and non-BFR high-load (Panel C) resistance exercise modalities similarly increased internal carotid artery blood flow from pre-exercise (●) to post-exercise (ס). Additionally, the males and females exhibited similar increases for each modality.
4. Discussion
The primary aim of the current study was to examine sex-specific differences in the magnitude of increases in ICA BF and cognitive executive performance in response to three distinct, volume-matched, RE modalities. These included low load-BFR, low load-non-BFR, and high load-non-BFR conditions applied during acute bilateral leg extension RE. Our main findings consisted of the following: (1) The females exhibited significantly greater improvements in cognitive flexibility and executive function index from baseline to post-exercise compared to the males independent of RE modality; (2) surprisingly, ICA BF and levels of arousal were not affected by RE modalities; and (3) as expected [23,[39], [40], [41], [42],62], the females demonstrated an attenuated exercise pressor reflex as demarcated by the smaller increases in MAP across each RE modality compared to the males. The novelty of our findings includes the investigation of RE as an approach to acutely improve cognition because the majority of the related studies have focused on aerobic modalities (e.g., cycling and running) [5,15,34]. Further, low load-BFR+RE has emerged as an exciting hypothetical technique to augment anticipated exercise-induced cognitive benefits [20,21,63], yet empirical data supporting this hypothesis remains particularly limited [22]. Although the current findings provide some evidence to support RE as a tool for positively influencing cognition, it should be noted that our results were derived from healthy young adults and acute exercise, which may yield different conclusions compared to chronic training in clinical and/or aged populations. Collectively, these results may inform future clinical trials aimed at preventing Alzheimer's Disease and related dementias such that RE does acutely, positively influence cognition, especially in females. These acute benefits were meaningful due to previous, relevant results suggesting acute changes may predict the magnitude of chronic benefits/adaptations [48] as well as that young, healthy individuals even demonstrated the capacity for cognitive enhancement [19]. Below, the interpretations of the results are discussed in the context of previous, relevant literature.
The present findings indicated that the females demonstrated an average ∼7.5-point increase in cognitive flexibility and executive function index regardless of RE modality, whereas the males exhibited an average ∼3-point decrease following the acute exercises. The reported cognitive values were standardized scores that were normalized from the raw values by the CNS Vital Signs software [58]. The cognitive flexibility domain score was generated from SAT correct responses minus SAT errors minus Stroop Test commission errors, while the executive function index was defined as SAT correct responses minus SAT errors. Based on the similar inputs, it was not surprising to observe matching cognitive flexibility and executive function index results. Furthermore, this observed advantage for the females compared to the males was in agreement with a previous robust systematic review and meta-analysis that clearly showed aerobic, resistance, and multimodal training were all associated with greater executive function improvements for females compared to males [34]. This previous conclusion [34] can now be extended to include specific variations of RE, including BFR and loads at opposing ends of the load spectrum. A possible explanation of the superior exercise-induced cognitive benefits garnered by females includes the influence of sex steroid hormones such that estradiol and testosterone have both been implicated in cognitive function [34,36]. There is developing evidence that females may possess a greater ability than males to upregulate neurotrophic and neuroplastic factors associated with the hypothesized exercise-induced link between sex hormones and cognition [8,14,34,64]. However, additional investigations are needed to determine the precise biological underpinning of the sex-specific influence of various hormones on cognitive function. Another non-mutually exclusive explanation consists of the notion that females may be more sensitive to fluctuations in BDNF [34,35,65,66], a neurotrophin that is involved in modulating neurological function, including neurogenesis and neuroprotection [9,12,67]. Although the current study examined acute responses to exercise, not chronic training, a previous review highlighted the potential of acute exercise-related increases in BDNF to influence subsequent improvements in cognition [68]. Further, it is possible that factors other than BDNF, such as insulin-like growth factor 1, contributed to these responses as well [69]. Notably, however, the current study did not report an exercise modality specific change in cognition for the males or females, yet previous studies have shown that BFR+RE increases blood lactate to a greater extent than non-BFR+RE with a matching load [21,70] in young (∼30 yrs old) males. This is important due to the link between lactate production and BDNF release [71,72]. Taken together, it was hypothesized that in the current study, there was likely a greater increase in plasma BDNF following the low load-BFR+RE at least compared to the low load-non-BFR condition, but these two conditions demonstrated similar cognitive changes, which presumably challenges the connection between increases in BDNF and acute cognitive enhancement. Thus, the precise cause for the similar cognitive changes among all three RE modalities remains unknown and speculative. Additional explanations may exist, but to be clear, the current participants were young, healthy adults, which eliminates the potential effects of longer exposure to sex hormones across the lifespan as well as the detriments associated with predictable age-related changes in hormonal profiles (e.g., menopause). Future work is still needed to tease apart mechanisms promoting changes in cognition following RE, especially the potential mechanisms associated with BFR+RE.
It was initially hypothesized that a particular RE modality would elicit a notable difference in acute cognitive performance and that this difference may be reflected by changes in ICA BF. This hypothesis was not completely supported. Currently, a majority of the participants (males and females) exhibited an increase in ICA BF immediately following each RE bout. As stated above, only the females exhibited acute cognitive improvements, and the mechanism responsible for this sex-specific response is currently not understood. The increase in ICA BF (i.e., CBF) for most of the participants (males and females) following the RE, however, was well supported by previous research. Previously, it was demonstrated that middle cerebral artery mean velocity increased 17 % shortly (15–30 s) after completing 10 bilateral leg press muscle actions with a load corresponding to 75 % 1RM [73]. In agreement, Koch et al. [74] also reported that during recovery from two exhaustive sets of dynamic leg flexion muscle actions, middle cerebral artery blood velocity substantially (15–30 %) increased. It was surprising that we did not observe a load-specific change in ICA BF, as previously 30 min of aerobic exercise at 70 % aerobic capacity elicited a greater increase in ICA BF immediately following the bout compared to the 30 % of maximum condition [6,47]. These previous studies reported increases in CBF, but unfortunately, none of these examples included cognitive measures. It should be noted that the role of acutely increased CBF in provoking exercise-induced cognitive improvements has been challenged. Ogoh et al. [30] reported that augmenting CBF via hypercapnia during rest and moderate aerobic exercise did not cause superior cognitive performance (Stroop Test) compared to exercise in normal room air (i.e., non-augmented CBF). There may be an exercise-type related effect such that aerobic and RE induce different downstream effects in response to increased CBF. This hypothesis remains largely speculative as the majority of available evidence in this regard has utilized aerobic exercise models, not RE [5,14,15,18,60]. Furthermore, it remains intriguing as to why there were no observed ICA BF differences between the load-matching BFR and non-BFR conditions [20,21]. Morita et al. [29] reported that BFR+RE with a similar repetition scheme as described in the current methodology (i.e., 1 × 30, 3 × 15) increased cerebral oxygenated hemoglobin to a greater extent than a volume-matched non-BFR condition. This previous study [29] utilized a unilateral forearm flexion exercise model with a load of 20 % 1RM, and thus, perhaps under these previous conditions, the smaller muscle mass and lower exercise load were insufficient to provoke measurable cerebral oxygenation differences without the application of BFR. Said differently, perhaps the heavier load in all of the conditions as well as the greater amount of recruited skeletal muscle mass in the current study compared to the study of Morita et al. [29] explains the discrepancy between our results. Hypothetically, the heavier loads and larger motor unit recruitment demands led to greater CBF and cerebral activity. It is possible that there are diminishing returns in terms of the cognitive benefits garnered from acute RE, and thus, perhaps the current conditions yielded the maximum RE-related cognitive enhancement response. Again, the previous findings of Ogoh et al. [30] should be noted such that the ability of increased CBF to influence cognition acutely remains questionable, and cognitive enhancement may be more related to increases in neuronal activation. Like ICA BF, however, the current study indicated no differences in the exercise-induced increases in the self-reported FAS values suggesting that arousal responded similarly to all conditions. Therefore, these collective results indicated that the RE conditions provoked parallel changes in CBF and arousal that seemingly were only beneficial for the female participants.
Routinely under a wide range of experimental conditions, a sex difference is observed in the magnitude of the exercise pressor reflex such that females tend to exhibit an attenuated response compared to males. Here, the males increased MAP from pre- to post-exercise (collapsed across RE modalities) by ∼18 points, whereas the females only increased by 11 points. Our research group has previously reported similar findings with independent samples following static handgrip [41] and BFR+RE with different cuff pressures [23]. We previously further examined this response by statistically controlling for body mass index (BMI), resting systolic BP, exercise performance (e.g., time to failure), and muscular strength, yet this analysis did not eliminate the female-related exercise pressor reflex attenuation [41]. Thus, based on previous investigations [39,40,42,62,75,76], we have attributed the difference in MAP increases to sex-specific muscle morphology and substrate utilization that likely lead to discrepancies in metabolic by-product accumulation, yielding variability in the stimulus for the exercise pressor reflex. Most recently, however, Tharpe et al. [40] reported that matching males and females (tested during days 1–5 of the menstrual cycle) on the basis of muscular strength eliminated the sex-specific exercise pressor reflex during a handgrip task. This was a very interesting finding due to a previous report suggesting that muscle strength accounts for less than 20 % of the variance in BP response to handgrip tasks [42], whereas the majority of the effect may be explained by factors associated with metabolite production [40]. Furthermore, Smith et al. [39] stated, “…available evidence suggests that the normal fluctuations in sex hormones across the menstrual cycle appear to have a limited role in modulating the exercise pressor reflex in pre-menopausal women,” (p.877). This offers additional support that factors intrinsic to the skeletal muscle (i.e., metabolite production) substantially influence the exercise pressor reflex in comparison to other aspects such as hormonal changes. Although it was not a primary aim of the current study to determine the influence of MAP changes on cognitive function, it is possible that the attenuated response exhibited by the females interacted with other physiological factors to promote cognitive enhancement. However, limited data exists directly linking acute RE-induced MAP responses to changes in cognition, especially in young, healthy adults [77,78]. Additional studies remain needed to fully understand the potential effect of sex-specific exercise pressor reflex responses on acute exercise provoked cognitive improvements.
Readers of the present investigation should consider the following experimental considerations when evaluating the currently provided interpretations of the results. Firstly, baseline cognitive values were derived from three run-in trials, but experts may suggest a need for additional run-in/practice trials. In an attempt to circumvent this potential issue, we defined the maximum value observed during these three practice trials as baseline cognition, which was likely the most conservative approach and placed the greatest burden of evidence on the RE experimental visits. Related to the cognitive measures, assessing other domains in addition to executive function index, cognitive flexibility, and reaction time may have been preferred by some readers. However, the current investigative team prioritized tests associated with executive function due to the amount of previous evidence strongly suggesting that this domain is sensitive to acute exercise. The next consideration includes the physiological measures only being collected at pre- and post-exercise. That is, no measures (e.g., MAP, ICA BF, FAS) were collected during the RE bouts. Additional measures of peak exercise MAP and magnitude of recovery may have provided further context for the provided interpretations. This also includes a lack of other time points following each RE condition, and thus, no data describing possible differences in sustained, elevated CBF are currently available. As previously mentioned, we did not control for the menstrual cycle or use of pharmaceutical contraception, which may be viewed as a limitation. This viewpoint is respected. However, based on the currently available evidence, it does not appear that the likely variation in menstrual cycle phases among the female participants substantially affected the current results [39] such that “…the consensus view is that the impact of the menstrual cycle and oral contraception use on various aspects of physiology is small or nonexistent,” (p.1284) [51]. It is also possible that the current study was underpowered to detect an interaction in the Sex × Exercise Modality ANOVA models. Related to our statistical approach, we did not statistically control for baseline differences (e.g., blood pressure) via the inclusion of various covariates, which may be viewed as a limitation as well. With great interest and additional resources, we look forward to increasing the sample size of our future studies.
In conclusion, the current results suggested that there were no measured differences among acute RE with the following conditions: low load-BFR, low load-non-BFR, and high load-non-BFR. That is, neither the application of BFR nor the use of a low or high load specifically influenced changes in ICA BF or cognition. Although the majority of participants exhibited increases in ICA BF in response to each RE condition, only the females presented improvements in executive function index and cognitive flexibility. Surprisingly, the males displayed a mean decrease in these cognitive measures. Possible explanations included the effects of sex-based hormones (e.g., estradiol), but notably, arousal (as measured by the FAS scale) did not vary between the males and females. It also remains possible that the sex-specific BP responses (i.e., exercise pressor reflex) contributed to the observed differences in cognition, but further research is necessary to more completely understand the prevalence and magnitude of this association. Again, the current study was conducted with young, healthy adults, but based on previous evidence [8,14,34,35], we hypothesize that the current sex differences would be present in an older population (+65 years old) and may even be more pronounced. With great enthusiasm, we look forward to following up the current investigation with chronic exercise training studies, especially those that further investigate the potential of BFR exercises to potentially augment cognitive benefits with lower loads (e.g., ≤30 %1RM). This may lead to an effective approach to boost exercise adherence in a diverse group of individuals.
CRediT authorship contribution statement
Genevieve B. Batman: Writing – review & editing, Writing – original draft, Funding acquisition, Formal analysis, Data curation, Conceptualization. Christian B. Cooper: Writing – review & editing, Investigation, Formal analysis, Data curation. Miranda K. Traylor: Writing – review & editing, Data curation, Conceptualization. Kyndall V. Ransom: Writing – review & editing, Data curation, Conceptualization. Ethan C. Hill: Writing – review & editing, Writing – original draft, Formal analysis, Conceptualization. Benjamin D. Hill: Writing – review & editing, Software, Resources, Project administration, Formal analysis, Conceptualization. Joshua L. Keller: Writing – review & editing, Writing – original draft, Visualization, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We want to thank the participants for volunteering to adhere to the study protocols. We would also like to thank the University of South Alabama's Summer Undergraduate Research Fellowship (GBB), the American Physiological Society's Summer Undergraduate Research Fellowship (KVR), and the College of Medicine's Summer Research Program (CBC). A special thanks to CNS Vital Signs for free access to their testing services. The work and assistance related to this investigation performed by Nicholas R. Cooper and Jeremy T. Herren are also acknowledged and greatly appreciated.
References
- 1.Saiyasit N., Butlig E.A.R., Chaney S.D., Traylor M.K., Hawley N.A., Randall R.B., et al. Neurovascular dysfunction in diverse communities with health disparities-contributions to dementia and Alzheimer's disease. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.915405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L.Y., Pei J., Zhan Y.J., Cai Y.W. Overview of meta-analyses of five non-pharmacological interventions for Alzheimer's disease. Front. Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.594432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings J., Lee G., Ritter A., Sabbagh M., Zhong K. Alzheimer's disease drug development pipeline: 2019. Alzheimer's Dement. 2019;5:272–293. doi: 10.1016/j.trci.2019.05.008. : Translational Research & Clinical Interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ransom K.V., Traylor M.K., Batman G.B., Mulekar M.S., Hill B.D., Nelson A.R., et al. Arterial stiffness mediates the association between age and processing speed at low levels of microvascular function in humans across the adult lifespan. Am. J. Physiol.-Heart Circ. Physiol. 2023 doi: 10.1152/ajpheart.00662.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boa Sorte Silva N.C., Gill D.P., Petrella RJ. A scoping review of multiple-modality exercise and cognition in older adults: limitations and future directions. Curr. Sports Med. Rep. 2020;19:298. doi: 10.1249/JSR.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 6.Barnes J.N., Burns J.M., Bamman M.M., Billinger S.A., Bodine S.C., Booth F.W., et al. Proceedings from the albert charitable trust inaugural workshop on ‘understanding the acute effects of exercise on the brain. Brain Plast. 2022;Preprint:1–16. doi: 10.3233/BPL-220146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes J.N., Corkery AT. Exercise Improves Vascular function, but does this translate to the brain? Brain Plast. 2018;4:65–79. doi: 10.3233/BPL-180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barha C.K., Liu-Ambrose T. Exercise and the Aging Brain: considerations for sex differences. Brain Plast. 2018;4:53–63. doi: 10.3233/BPL-180067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh E.I., Smith L., Northey J., Rattray B., Cherbuin N. Towards an understanding of the physical activity-BDNF-cognition triumvirate: A review of associations and dosage. Ageing Res. Rev. 2020;60 doi: 10.1016/j.arr.2020.101044. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen BK. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 2019:1. doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- 11.Umegaki H., Sakurai T., Arai H. Active life for brain health: a narrative review of the mechanism underlying the protective effects of physical activity on the brain. Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.761674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traylor M.K., Bauman A.J., Saiyasit N., Frizell C.A., Hill B.D., Nelson A.R., et al. An examination of the relationship among plasma brain derived neurotropic factor, peripheral vascular function, and body composition with cognition in midlife African Americans/Black individuals. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.980561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidoni E.D., Johnson D.K., Morris J.K., Sciver A.V., Greer C.S., Billinger S.A., et al. Dose-response of aerobic exercise on cognition: a community-based, pilot randomized controlled trial. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barha C.K., Hsiung G.Y.R., Best J.R., Davis J.C., Eng J.J., Jacova C., et al. Sex difference in aerobic exercise efficacy to improve cognition in older adults with vascular cognitive impairment: secondary analysis of a randomized controlled trial. J. Alzheimers Dis. 2017;60:1397–1410. doi: 10.3233/JAD-170221. [DOI] [PubMed] [Google Scholar]
- 15.Liu-Ambrose T., Donaldson MG. Exercise and cognition in older adults: is there a role for resistance training programmes? Br. J. Sports Med. 2009;43:25–27. doi: 10.1136/bjsm.2008.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landrigan J.F., Bell T., Crowe M., Clay O.J., Mirman D. Lifting cognition: a meta-analysis of effects of resistance exercise on cognition. Psychol. Res. 2020;84:1167–1183. doi: 10.1007/s00426-019-01145-x. [DOI] [PubMed] [Google Scholar]
- 17.De la Rosa A., Olaso-Gonzalez G., Arc-Chagnaud C., Millan F., Salvador-Pascual A., García-Lucerga C., et al. Physical exercise in the prevention and treatment of Alzheimer's disease. J. Sport Health Sci. 2020;9:394–404. doi: 10.1016/j.jshs.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anders J.P.V., Kraemer W.J., Newton R.U., Post E.M., Caldwell L.K., Beeler M.K., et al. Acute effects of high-intensity resistance exercise on cognitive function. J. Sports Sci. Med. 2021;20:391–397. doi: 10.52082/jssm.2021.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moffitt T.E., Belsky D.W., Danese A., Poulton R., Caspi A. The longitudinal study of aging in human young adults: knowledge gaps and research agenda. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72:210–215. doi: 10.1093/gerona/glw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Törpel A., Herold F., Hamacher D., Müller N.G., Schega L. Strengthening the brain—is resistance training with blood flow restriction an effective strategy for cognitive improvement? J. Clin. Med. 2018;7:337. doi: 10.3390/jcm7100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada Y., Frith E.M., Wong V., Spitz R.W., Bell Z.W., Chatakondi R.N., et al. Acute exercise and cognition: A review with testable questions for future research into cognitive enhancement with blood flow restriction. Med. Hypotheses. 2021;151 doi: 10.1016/j.mehy.2021.110586. [DOI] [PubMed] [Google Scholar]
- 22.Sardeli A.V., Ferreira M.L.V., Santos L do C., Rodrigues M de S., Damasceno A., Cavaglieri C.R., et al. Low-load resistance exercise improves cognitive function in older adults. Rev. Bras. Med. Esporte. 2018;24:125–129. doi: 10.1590/1517-869220182402179200. [DOI] [Google Scholar]
- 23.Gray S., Cuomo A., Proppe C.E., Traylor M.K., Hill E.C., Keller JL. Effects of sex and cuff pressure on physiological responses during blood flow restriction resistance exercise in young adults. Med. Sci. Sports Exerc. 2023;55:920–931. doi: 10.1249/MSS.0000000000003103. [DOI] [PubMed] [Google Scholar]
- 24.Hill E.C., Housh T.J., Keller J.L., Smith C.M., Schmidt R.J., Johnson GO. Early phase adaptations in muscle strength and hypertrophy as a result of low-intensity blood flow restriction resistance training. Eur. J. Appl. Physiol. 2018;118:1831–1843. doi: 10.1007/s00421-018-3918-8. [DOI] [PubMed] [Google Scholar]
- 25.Seals DR. Influence of muscle mass on sympathetic neural activation during isometric exercise. J. Appl. Physiol. 1989;67:1801–1806. doi: 10.1152/jappl.1989.67.5.1801. (1985) [DOI] [PubMed] [Google Scholar]
- 26.Franke W.D., Boettger C.F., McLean SP. Effects of varying central command and muscle mass on the cardiovascular responses to isometric exercise. Clin. Physiol. 2000;20:380–387. doi: 10.1046/j.1365-2281.2000.00273.x. [DOI] [PubMed] [Google Scholar]
- 27.Thomas K., Goodall S., Howatson G. Performance fatigability is not regulated to a peripheral critical threshold. Exerc. Sport Sci. Rev. 2018;46:240–246. doi: 10.1249/JES.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 28.Estrada J.A., Ducrocq G.P., Kaufman MP. The magnitude of the exercise pressor reflex is influenced by the active skeletal muscle mass in the decerebrate rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019 doi: 10.1152/ajpregu.00263.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morita T., Fukuda T., Kikuchi H., Ikeda K., Yumoto M., Sato Y. Effects of blood flow restriction on cerebral blood flow during a single arm-curl resistance exercise. Int. J. KAATSU Train. Res. 2010;6:9–12. doi: 10.3806/ijktr.6.9. [DOI] [Google Scholar]
- 30.Ogoh S., Tsukamoto H., Hirasawa A., Hasegawa H., Hirose N., Hashimoto T. The effect of changes in cerebral blood flow on cognitive function during exercise. Physiol. Rep. 2014;2:e12163. doi: 10.14814/phy2.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellström G., Fischer-Colbrie W., Wahlgren N.G., Jogestrand T. Carotid artery blood flow and middle cerebral artery blood flow velocity during physical exercise. J. Appl. Physiol. 1996;81:413–418. doi: 10.1152/jappl.1996.81.1.413. (1985) [DOI] [PubMed] [Google Scholar]
- 32.Davenport M.H., Hogan D.B., Eskes G.A., Longman R.S., Poulin MJ. Cerebrovascular reserve: the link between fitness and cognitive function? Exerc. Sport Sci. Rev. 2012;40:153–158. doi: 10.1097/JES.0b013e3182553430. [DOI] [PubMed] [Google Scholar]
- 33.Olivo G., Nilsson J., Garzón B., Lebedev A., Wåhlin A., Tarassova O., et al. Immediate effects of a single session of physical exercise on cognition and cerebral blood flow: A randomized controlled study of older adults. Neuroimage. 2021;225 doi: 10.1016/j.neuroimage.2020.117500. [DOI] [PubMed] [Google Scholar]
- 34.Barha C.K., Davis J.C., Falck R.S., Nagamatsu L.S., Liu-Ambrose T. Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocrinol. 2017;46:71–85. doi: 10.1016/j.yfrne.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Barha C.K., Liu-Ambrose T., Best J.R., Yaffe K., Rosano C. Sex-dependent effect of the BDNF Val66Met polymorphism on executive functioning and processing speed in older adults: evidence from the health ABC study. Neurobiol. Aging. 2019;74:161–170. doi: 10.1016/j.neurobiolaging.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurvich C., Thomas N., Kulkarni J., Lanzenberger R., Kranz G.S., Savic I. Handbook of Clinical Neurology. Elsevier; 2020. Chapter 7 - Sex differences in cognition and aging and the influence of sex hormones; pp. 103–115. vol. 175. [DOI] [PubMed] [Google Scholar]
- 37.Colcombe S., Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 38.Zeller N.P., Miller K.B., Zea R.D., Howery A.J., Labrecque L., Aaron S.E., et al. Sex-specific effects of cardiorespiratory fitness on age-related differences in cerebral hemodynamics. J. Appl. Physiol. 2022;132:1310–1317. doi: 10.1152/japplphysiol.00782.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith J.R., Koepp K.E., Berg J.D., Akinsanya J.G., Olson TP. Influence of sex, menstrual cycle, and menopause status on the exercise pressor reflex. Med. Sci. Sports Exerc. 2019;51:874–881. doi: 10.1249/MSS.0000000000001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tharpe M.A., Linder B.A., Babcock M.C., Watso J.C., Pollin K.U., Hutchison Z.J., et al. Adjusting for muscle strength and body size attenuates sex differences in the exercise pressor reflex in young adults. Am. J. Physiol. Heart Circ. Physiol. 2023 doi: 10.1152/ajpheart.00151.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keller J.L., Kennedy K.G., Hill E.C., Fleming S.R., Colquhoun R.J., Schwarz NA. Handgrip exercise induces sex-specific mean arterial pressure and oxygenation responses but similar performance fatigability. Clin. Physiol. Funct. Imaging. 2022;42:127–138. doi: 10.1111/cpf.12739. [DOI] [PubMed] [Google Scholar]
- 42.Lee J.B., Notay K., Seed J.D., Nardone M., Omazic L.J., Millar PJ. Sex differences in muscle metaboreflex activation following static handgrip exercise. Med. Sci. Sports Exerc. 2021;53:2596–2604. doi: 10.1249/MSS.0000000000002747. [DOI] [PubMed] [Google Scholar]
- 43.Amann M., Wan H.Y., Thurston T.S., Georgescu V.P., Weavil JC. On the influence of group III/IV muscle afferent feedback on endurance exercise performance. Exerc. Sport Sci. Rev. 2020;48:209–216. doi: 10.1249/JES.0000000000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaufman M.P., Hayes SG. The exercise pressor reflex. Clin. Auton. Res. 2002;12:429–439. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- 45.Ducrocq G.P., Anselmi L., Ruiz-Velasco V., Kaufman M.P. Lactate and hydrogen ions play a predominant role in evoking the exercise pressor reflex during ischaemic contractions but not during freely perfused contractions. J. Physiol. 2024 doi: 10.1113/JP286488. n.d.;n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Counts B.R., Rossow L.M., Mattocks K.T., Mouser J.G., Jessee M.B., Buckner S.L., et al. Let's talk about sex: where are the young females in blood flow restriction research? Clin. Physiol. Funct. Imaging. 2018;38:1–3. doi: 10.1111/cpf.12394. [DOI] [PubMed] [Google Scholar]
- 47.Moir M.E., Corkery A.T., Apfelbeck A.A., Pearson A.G., Loggie N.A., Miller K.B., et al. Acute post-exercise internal carotid artery hemodynamics are intensity dependent in young adults. Physiology. 2023;38 doi: 10.1152/physiol.2023.38.S1.5731656. [DOI] [Google Scholar]
- 48.Voss M.W., Weng T.B., Narayana-Kumanan K., Cole R.C., Wharff C., Reist L., et al. Acute exercise effects predict training change in cognition and connectivity. Med. Sci. Sports Exerc. 2020;52:131–140. doi: 10.1249/MSS.0000000000002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKay A.K.A., Stellingwerff T., Smith E.S., Martin D.T., Mujika I., Goosey-Tolfrey V.L., et al. Defining training and performance caliber: A participant classification framework. Int. J. Sports Physiol. Perform. 2022;17:317–331. doi: 10.1123/ijspp.2021-0451. [DOI] [PubMed] [Google Scholar]
- 50.Blagrove R.C., Bruinvels G., Pedlar CR. Variations in strength-related measures during the menstrual cycle in eumenorrheic women: A systematic review and meta-analysis. J. Sci. Med. Sport. 2020;23:1220–1227. doi: 10.1016/j.jsams.2020.04.022. [DOI] [PubMed] [Google Scholar]
- 51.D'Souza A.C., Wageh M., Williams J.S., Colenso-Semple L.M., McCarthy D.G., McKay A.K.A., et al. Menstrual cycle hormones and oral contraceptives: a multimethod systems physiology-based review of their impact on key aspects of female physiology. J. Appl. Physiol. 2023;135:1284–1299. doi: 10.1152/japplphysiol.00346.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minson C.T., Halliwill J.R., Young T.M., Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 53.Williams J.S., Dunford E.C., MacDonald MJ. Impact of the menstrual cycle on peripheral vascular function in premenopausal women: systematic review and meta-analysis. Am. J. Physiol. Heart. Circ. Physiol. 2020;319:H1327–H1337. doi: 10.1152/ajpheart.00341.2020. [DOI] [PubMed] [Google Scholar]
- 54.Tinsley G.M., Moore M.L., Benavides M.L., Dellinger J.R., Adamson BT. 3-Dimensional optical scanning for body composition assessment: A 4-component model comparison of four commercially available scanners. Clin. Nutr. 2020;39:3160–3167. doi: 10.1016/j.clnu.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Dora K., Suga T., Tomoo K., Sugimoto T., Mok E., Tsukamoto H., et al. Similar improvements in cognitive inhibitory control following low-intensity resistance exercise with slow movement and tonic force generation and high-intensity resistance exercise in healthy young adults: a preliminary study. J. Physiol. Sci. 2021;71:22. doi: 10.1186/s12576-021-00806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McEwen J.A., Owens J.G., Jeyasurya J. Why is it crucial to use personalized occlusion pressures in blood flow restriction (BFR) rehabilitation? J. Med. Biol. Eng. 2019;39:173–177. doi: 10.1007/s40846-018-0397-7. [DOI] [Google Scholar]
- 57.Bastos V., Rodrigues F., Davis P., Teixeira DS. Assessing affective valence and activation in resistance training with the feeling scale and the felt arousal scale: A systematic review. PLoS One. 2023;18 doi: 10.1371/journal.pone.0294529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gualtieri C.T., Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS vital signs. Arch. Clin. Neuropsychol. 2006;21:623–643. doi: 10.1016/j.acn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 59.Lambourne K., Tomporowski P. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Res. 2010;1341:12–24. doi: 10.1016/j.brainres.2010.03.091. [DOI] [PubMed] [Google Scholar]
- 60.Chang Y.K., Labban J.D., Gapin J.I., Etnier JL. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012;1453:87–101. doi: 10.1016/j.brainres.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 61.Yamada Y., Kataoka R., Bell Z.W., Wong V., Spitz R.W., Song J.S., et al. Improved interference control after exercise with blood flow restriction and cooling is associated with but not mediated by increased lactate. Physiol. Behav. 2023;270 doi: 10.1016/j.physbeh.2023.114291. [DOI] [PubMed] [Google Scholar]
- 62.Notay K., Lee J.B., Incognito A.V., Seed J.D., Arthurs A.A., Millar PJ. Muscle Strength Influences Pressor Responses to Static Handgrip in Men and Women. Med. Sci. Sports Exerc. 2018;50:778–784. doi: 10.1249/MSS.0000000000001485. [DOI] [PubMed] [Google Scholar]
- 63.Cahalin L.P., Formiga M.F., Anderson B., Cipriano G., Hernandez E.D., Owens J., et al. A call to action for blood flow restriction training in older adults with or susceptible to sarcopenia: A systematic review and meta-analysis. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.924614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aizawa K., Iemitsu M., Otsuki T., Maeda S., Miyauchi T., Mesaki N. Sex differences in steroidogenesis in skeletal muscle following a single bout of exercise in rats. J. Appl. Physiol. 2008;104:67–74. doi: 10.1152/japplphysiol.00558.2007. (1985) [DOI] [PubMed] [Google Scholar]
- 65.Komulainen P., Pedersen M., Hänninen T., Bruunsgaard H., Lakka T.A., Kivipelto M., et al. BDNF is a novel marker of cognitive function in ageing women: the DR's EXTRA Study. Neurobiol. Learn. Mem. 2008;90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 66.Bakos J., Hlavacova N., Rajman M., Ondicova K., Koros C., Kitraki E., et al. Enriched environment influences hormonal status and hippocampal brain derived neurotrophic factor in a sex dependent manner. Neuroscience. 2009;164:788–797. doi: 10.1016/j.neuroscience.2009.08.054. [DOI] [PubMed] [Google Scholar]
- 67.Fortune J.M., Kelly Á.M., Robertson I.H., Hussey J. An investigation into the relationship between cardiorespiratory fitness, cognition and BDNF in young healthy males. Neurosci. Lett. 2019;704:126–132. doi: 10.1016/j.neulet.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 68.Piepmeier A.T., Etnier JL. Brain-derived neurotrophic factor (BDNF) as a potential mechanism of the effects of acute exercise on cognitive performance. J. Sport Health Sci. 2015;4:14–23. doi: 10.1016/j.jshs.2014.11.001. [DOI] [Google Scholar]
- 69.Cassilhas R.C., Lee K.S., Fernandes J., Oliveira M.G.M., Tufik S., Meeusen R., et al. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–317. doi: 10.1016/j.neuroscience.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 70.Fujita S., Abe T., Drummond M.J., Cadenas J.G., Dreyer H.C., Sato Y., et al. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J. Appl. Physiol. 2007;103:903–910. doi: 10.1152/japplphysiol.00195.2007. [DOI] [PubMed] [Google Scholar]
- 71.Müller P., Duderstadt Y., Lessmann V., Müller NG. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020;9:1136. doi: 10.3390/jcm9041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El Hayek L., Khalifeh M., Zibara V., Abi Assaad R., Emmanuel N., Karnib N., et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF) J. Neurosci. 2019;39:2369–2382. doi: 10.1523/JNEUROSCI.1661-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edwards M.R., Martin D.H., Hughson RL. Cerebral hemodynamics and resistance exercise. Med. Sci. Sports Exerc. 2002;34:1207. doi: 10.1097/00005768-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 74.Koch A., Ivers M., Gehrt A., Schnoor P., Rump A., Rieckert H. Cerebral autoregulation is temporarily disturbed in the early recovery phase after dynamic resistance exercise. Clin. Auton. Res. 2005;15:83–91. doi: 10.1007/s10286-005-0249-8. [DOI] [PubMed] [Google Scholar]
- 75.Haizlip K.M., Harrison B.C., Leinwand LA. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology. 2015;30:30–39. doi: 10.1152/physiol.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Montero D., Madsen K., Meinild-Lundby A.K., Edin F., Lundby C. Sexual dimorphism of substrate utilization: differences in skeletal muscle mitochondrial volume density and function. Exp. Physiol. 2018;103:851–859. doi: 10.1113/EP087007. [DOI] [PubMed] [Google Scholar]
- 77.Washio T., Suzuki K., Saito S., Watanabe H., Ando S., Brothers R.M., et al. Effects of acute interval handgrip exercise on cognitive performance. Physiol. Behav. 2021;232 doi: 10.1016/j.physbeh.2021.113327. [DOI] [PubMed] [Google Scholar]
- 78.Palmiere S., Wade M., DeBlois J.P., Lefferts W.K., Heffernan KS. Aortic stiffness, central pulse pressure and cognitive function following acute resistance exercise. Eur. J. Appl. Physiol. 2018;118:2203–2211. doi: 10.1007/s00421-018-3948-2. [DOI] [PubMed] [Google Scholar]