Abstract
Japanese encephalitis virus (JEV) is associated with encephalitis in humans and reproductive and neurological illness in pigs. JEV has expanded beyond its native distribution in southeast Asia, with identifications in Europe (2010) and Africa (2016), and most recently, its spread into mainland Australia (2021−2022). The introduction of JEV into the United States (US) is a public health risk, and could also impact animal health and the food supply. To efficiently and cost-effectively manage risk, a better understanding of how and where diseases will be introduced, transmitted, and spread is required. To achieve this objective, we updated our group's previous qualitative risk assessment using an established semi-quantitative risk assessment tool (MINTRISK) to compare the overall rate of introduction and risk, including impacts, of JEV in seven US regions. The rate of introduction from the current region of distribution was considered negligible for the Northeast, Midwest, Rocky Mountain, West, Alaska, and Hawaii regions. The South region was the only region with a pathway that had a non-negligible rate of introduction; infected mosquito eggs and larvae introduced via imported used tires (very low; 95% uncertainty interval (UI) = negligible to high). The overall risk estimate for the South was very high (95% UI = very low to very high). Based on this risk assessment, the South region should be prioritized for surveillance activities to ensure the early detection of JEV. The assumptions used in this risk assessment, due to the lack of information about the global movement of mosquitoes, number of feral pigs in the US, the role of non-ardeid wild birds in transmission, and the magnitude of the basic reproduction ratio of JEV in a novel region, need to be fully considered as these impact the estimated probability of establishment.
Keywords: Risk analysis, Japanese encephalitis, Emergence, United States, Flavivirus, Vector-borne
1. Introduction
Japanese encephalitis virus (JEV) is a mosquito-borne flavivirus and the causative agent of Japanese encephalitis (JE), one of the most significant human viral encephalitides in Asia [1]. This virus not only poses a public health concern in endemic/epidemic areas, but also an animal health and welfare risk, as it may cause encephalitis in horses, and reproductive and neurologic illness in boars/sows and piglets, respectively [2]. Japanese encephalitis virus is primarily maintained through wild vector-host cycles, with birds from the Ardeidae family (i.e., wading birds) acting as the main maintenance host and domestic swine acting as the amplifying host. Feral swine and other mammals, such as bats, are also susceptible to JEV infection and produce viral titers high enough to transmit JEV to mosquitoes [3]. However, there is evidence of vector-free transmission from challenge studies [4,5], but more research is needed to identify the effectiveness of this route relative to the vector-host cycles on virus maintenance. While Culex mosquitoes are considered the primary vectors, species from other genera, such as Aedes, as well as from areas free of JEV have been shown to be competent in experimental studies (e.g., US populations of Culiseta inornata and Aedes nigromaculis; see [6]).
Whereas JEV is endemic in Southeast Asia and areas of South Asia, and epidemic in East Asia, parts of Russia bordering the Sea of Japan, and Australia, there has been an isolated case in Africa (i.e., an autochthonous case in Angola in 2016 [7]) and viral detection in Europe (i.e., detected in birds (1997–2000; [8,9]) and Culex pipiens (2010; [10]) in Italy), indicating previous introduction and transmission events in novel areas. Most recently, Australia experienced an outbreak associated with genotype IV of JEV (2021–2022) that extended its range throughout mainland Australia from the previously identified area of the Torres Strait islands and the Northern Peninsula Area of Cape York (1995–2004) [11]. While the origin of this outbreak is unknown, a suite of competent vectors, maintenance and amplifying hosts, and conducive climatic events (i.e., La Niña rainfall events) facilitated transmission [[11], [12], [13]]. As JEV continues to expand its distribution, it is crucial to remain vigilant of other areas at risk for a JEV incursion, and the United States (US) is one such favorable area as demonstrated by the introduction and establishment of other mosquito-borne flaviviruses like West Nile virus (WNV). Given the presence of immunologically naïve populations of known maintenance and amplifying hosts, competent vectors, extensive travel and trade from JEV-affected countries, similar climatic and environmental conditions as endemic/epidemic regions, and the absence of active JEV surveillance, the incursion and establishment of JEV in the US could lead to disease outbreaks in humans and pigs. This would result in significant socio-ethical and economic impacts on human and animal health and welfare.
The risk of JEV introduction and transmission in the US has been largely addressed based on literature reviews [[14], [15], [16]], but to our knowledge only two papers have addressed this question using a structured risk assessment framework [17,18]. Qualitative, like quantitative, risk assessments provide necessary frameworks for decision-makers to assess risk and impact, however qualitative risk assessments are extremely beneficial when data are lacking and/or rapid decisions are required [19]. In a qualitative risk assessment of JEV introduction into the continental US, our team [18] determined the risk of JEV entry ranged from very high (i.e., infected mosquitoes via aircraft) to low/moderate (i.e., adult mosquitoes via ships/shipping containers). Upon evaluating these pathways further via a quantitative risk assessment, our model predicted a very high risk (0.95 median probability) of at least one infected mosquito (with a median of 3 infected mosquitoes) being introduced via aircraft from JEV-affected areas into the US every year, from March to October [17].
When estimating the probability of transmission using the qualitative risk assessment, it varied, being highly dependent on the idiosyncrasies of the region of entry; ultimately, with the complexity and fragility of stacking probabilities, we determined the probability of establishment as negligible with the existing weather and climatic conditions [18]. Considering the dependence of establishment on transmission and the uncertainty of its estimation due to the size and diversity of the geographic area assessed, we aimed to re-assess this qualitative risk assessment at a regional level, now including Alaska and Hawaii, rather than considering continental US as a single region.
To compare introduction risks and impacts between regions at risk, we used a semi-quantitative risk assessment tool [20] developed by Wageningen BioVeterinary Research and Wageningen Economic Research, and supported by the European Food Safety Authority (EFSA). Designed to assess the rate of introduction and risk of vector-borne diseases of livestock, the algorithms of this tool put strong emphasis on the vector-host-pathogen interactions when estimating probabilities. This tool has been used to evaluate the introduction risk of several pathogens transmitted by mosquitoes, Culicoides, ticks, and sand flies that transmit various pathogens affecting livestock, such as WNV, Rift Valley fever, African horse sickness, and epizootic hemorrhagic disease [[20], [21], [22]]. MINTRISK was developed as a generic and flexible risk assessment tool to provide a transparent and repeatable method that allows users to address the probability of entry, transmission, establishment, spread and persistence, but also evaluate the economic, environmental and socio-ethical impacts of a disease.
The objective of this study was to update and re-assess the 2018 qualitative risk assessment by Oliveira and colleagues [18] - which estimated the risk of emergence of JEV in the continental US - at a regional level by incorporating information regarding transmission post-emergence, establishment, and spread, based on the latest scientific information (i.e., literature and data sources), recent outbreak data from Australia, and expert opinion.
2. Materials and methods
Previously, our group utilized a Framework to assess Emerging VEctor-borne disease Risks (FEVER; [23]) to qualitatively assess the risk of an incursion of JEV into the US [18]. Building on this previous risk assessment, we utilized a Model for INTegrated Risk Assessment (MINTRISK; [20]), a model which incorporates FEVER, to perform a semi-quantitative risk assessment evaluating the risk of JEV introduction into seven US regions from the current region of distribution. Using user-determined parameters about virus entry, transmission, establishment, spread, persistence, and impact, MINTRISK calculates three summary output parameters: 1) the rate of introduction, 2) the estimated epidemic size, and 3) the overall risk estimate.
Utilizing the information from a systematic review on vector and host competence for JEV [24,25], current scientific literature, government and scientific databases, and expert opinion, the previous risk assessment's parameters and pathways for introduction were re-evaluated for seven US regions. Subsequently, the steps of spread, persistence, and impact were re-evaluated for regions with a non-negligible risk of introduction (i.e., a median risk score for rate of introduction >0). The sources of information and assumptions used to estimate the input parameters for the risk assessment are summarized in Table 1 and all input parameters are reported in the supplementary materials (S1). Below we describe the methods used to calculate the input parameters; for all specific MINTRISK calculations, please see [20]. Briefly, input parameters are entered by scoring a set of questions for each step, choosing from five qualitative answers which range from very low to very high; each of those categories are associated with a quantitative explanation (on a logarithmic scale) tailored to each question. In addition to inputting specific parameters, the level of uncertainty, classified as low, moderate, or high, for each question is also captured. Uncertainty is accounted for using Monte Carlo simulation, along a most likely value for each answer category. As such, qualitative inputs are converted into quantitative values that are considered when performing model calculations, which in turn are transformed into qualitative outputs.
Table 1.
A list of the sources and methods used and assumptions made to estimate the input parameters used in MINTRISK.
| Parameter | Methods and assumptions |
|---|---|
| Entry | |
| Annual volume of hosts / vectors moved along the pathway from the region of distribution to the region at risk | Mosquito eggs/larvae via imported tires and plants: The number of imported tires and plants in 2020 was estimated from the UNSD Commodity Trade database and multiplied by the average number of vectors collected in tires and imported plants as reported in the literature (see section 2.2.0 “Entry” for details) |
| Adult mosquitoes via aircraft: Estimated from the average number of non-stop flights from the region of distribution to a port within the region at risk from 2017 to 2022 multiplied by the average number of vectors collected in aircrafts as reported in the literature (see section 2.2.0 “Entry” for details) | |
| Adult mosquitoes via ships: Estimated from the average number of voyages with an origin port in the region of distribution to a port within the region at risk from 2017 to 2020, adjusted for the number of inspected ships positive for the presence of mosquitoes, multiplied by the average number of vectors collected in ships as reported in the literature (see section 2.2.0 “Entry” for details) | |
| Adult mosquitoes via containers: Estimated from the average yearly tonnage of cargo with an origin port in the region of distribution shipped to a port within the region at risk from 2017 to 2020, adjusted for the amount of imported foreign cargo that is containerized, and multiplied by the number of vectors collected in containers as reported in the literature (see section 2.2.0 “Entry” for details) | |
| Migrating ardeid birds: Estimated from [51,52] | |
| Probability of pathogen surviving in pathway until arrival to the region at risk | Mosquito eggs and larvae: Vertical transmission to F1 adults is 0.18% to 0.19% (Ae. albopictus) and to F1 larvae is 1:328 (Ae. alcasidi) and 1:2334 (Ae. vexans) [95] |
| Adult mosquitoes: Based on the length of JEV transmission in mosquitoes (7–34 days; [96]) and the average travel/shipping time from the regions of distribution (i.e., China and India) to the region at risk for the pathway [97] | |
| Migrating ardeid birds: [18] | |
| Probability of pathogen presence in pathway upon arrival despite control / preventive measures | Expert opinion |
| Prevalence of infection in the region of distribution in an endemic situation⁎ | Prevalence of vector and host infection from [24] and an update of this meta-analysis |
| Transmission | |
| Basic reproduction number (R0) | JEV swine-mosquito-swine: [[53], [54], [55]]; WNV wild bird-mosquito-mosquito: [56]; avian malaria wild bird-mosquito-wild bird: [57] |
| Fraction of the host population susceptible to infection | Assumed a completely naïve population |
| Distribution of the vector in the region at risk | Based on known distributions from [98] and habitat suitability for likely dominant U.S. vectors (Culex pipiens and Cx. quinquefasciatus; [99]) as well as expert opinion |
| Establishment | |
| Probability of first transmission step (from introduced infected vector to local host or introduced infected host to local vector) | Mosquito eggs/larvae and adults: Multiplicative probability of the survival to adulthood and biting, host overlap at the port of entry, and transmission rate of vectors (see section 2.2.2.0 “First transmission step” for details) |
| Migrating ardeid birds: Multiplicative probability of the overlap of migrant bird sightings with the mosquito season for the region and the infection rate of putative US vectors (see section 2.2.2.0 “First transmission step” for details) | |
| Probability of second transmission step (from local infected host to local vector or local infected vector to local host) | Mosquito eggs/larvae and adults: Determined by expert opinion accounting for information on mosquito density and the presence of mosquito habitats at ports of entry |
| Migrating ardeid birds: Multiplicative probability of the overlap of migrant bird sightings with the mosquito season for the region, the median mean relative abundance of ardeid species in the region, and the transmission rate of putative US vectors (see section 2.2.2.1 “Second transmission step” for details) | |
| Spread | |
| Dilution effect due to the presence of non-susceptible hosts | Based on the relative abundance of susceptible hosts (swine and ardeids) and non-susceptible hosts (predominantly cattle and humans) in the region and the feeding patterns of Culex quinquefasciatus, Cx. pipiens, and Cx. tarsalis (all with a stronger preference for birds, then mammals) and Aedes albopictus and Ae. japonicus (both with a preference for humans) |
| Effectiveness of vector and larval control in reducing the spread of infection | Expert opinion |
| Effectiveness of control measures of host animals in reducing the spread of the infection | None; No current National Animal Disease Preparedness and Response Plan for JEV |
| Number of infection generations per vector season | Expert opinion |
| Overlap between vector abundance and host density | Expert opinion |
| Inhibition of local spread by spatial effects | Unknown |
| Contribution of vectors to long-distance spread | Expert opinion |
| Contribution of host animals to long-distance spread | Although movement of domestic and feral pigs [72,100] was considered low, ardeids were assumed to contribute heavily to long distance dispersal, based on their role in the movement of WNV in the US (e.g., [101,102]) |
| Length of the vector season | Expert opinion |
| Population size of susceptible host animals | Based primarily on domestic pig inventory [27] and feral pig population [58], but the number of ardeid species found in the region and their relative abundance's [59] were also taken into account. |
| Persistence | |
| Overwintering via persistent infection of the host | Swine: [4,103]; Ardeids: Unknown |
| Overwintering via vertical transmission in the host | Fetuses infected through vertical transmission are generally aborted or result in stillbirths [88] |
| Overwintering via direct host-to-host transmission | Swine: [4,54,104]; Other hosts: Unknown |
| Overwintering via survival of an infected (adult) vector | Expert opinion |
| Overwintering via vertical transmission in the vector | Vertical transmission to F1 larvae in Aedes alcasidi, Ae. vexans, Armigeres subalbatus, Ar. flavus, Culex pipiens pallens, Cx. pipiens molestus Cx. quinquefasciatus and vertical transmission to F1 larvae and adults of Cx. annulus, Cx. tritaeniorhynchus [95] |
| Overwintering via other mechanisms | [105] |
| Impact | |
| Direct agricultural economic losses per host animal | Expert opinion from 2021/2022 Australian JEV outbreak |
| Indirect agricultural economic losses per host animal | Expert opinion from 2021/2022 Australian JEV outbreak |
| Economic losses due to human disease (per 100 animal hosts) | Unknown |
| Indirect agricultural economic losses for the region at risk due to presence of the disease | Expert opinion from 2021/2022 Australian JEV outbreak |
| Economic losses due to side effects | Unknown |
Information for both epidemic and endemic occurrence in the region of distribution was incorporated (see Table S2), however MINTRISK uses the parameter with the highest semi-quantitative score in the model calculations, which was endemic occurrence.
2.1. Advisory group and expert opinion
An advisory group was formed to provide support on several areas including the development and refinement of research questions and identification of outcomes that are relevant to the US swine industry, provision of feedback on data collection tools, expert opinion pertaining to assumptions and advice on sources of information, policy and/or procedures relevant to the US swine industry, and perspective into data interpretation; they were also asked to provide a forum to discuss, communicate and facilitate the dissemination of the study findings, and expert opinion on the latest JEV outbreak in Australia. Members (n = 17), which were selected with the assistance of US swine stakeholders, consisted of swine producers, veterinarians, entomologists, researchers and stakeholders from US and Australia.
To elicit expert opinion from our advisory group members, our team designed and implemented questionnaires, which were remotely delivered via the QualtricsXM platform (Qualtrics® International, Seattle, WA, USA). The questionnaires consisted of multiple-choice, fill-in-the-blank or short-essay questions about the pathways of introduction, transmission and establishment, as well as the prioritization of information when evaluating the role of pigs in the transmission of JEV. The questionnaire was divided into three sections: the first section pertained to JEV introduction pathways to the US, the second section referred to paths or mechanisms of transmission of the virus after a potential incursion while considering regional differences, and lastly, the third section included additional questions regarding prioritization of factors for assessing the role of pigs (domestic and feral) in the transmission of JEV in the US. The obtained data were exported from Qualtrics and summarized to then be incorporated as model parameters into the risk assessment models.
Lastly, we reached out to groups of individuals within and outside (n = 14) our advisory group (as per advisory group members' suggestions), with specific expertise (e.g., entomology, Australian outbreak, feral pig biology/management) to elicit expert opinion regarding likelihood of persistence and impact of disease. This information was requested via email, and upon receipt, collated to be inputted in the risk assessment models.
2.2. US regions at risk
Seven US regions at risk (Fig. 1, Fig. S1) were identified by grouping the 48 states of the contiguous US based on similar climate (i.e., Köppen-Geiger climate classification; [26]), county level density of domestic swine production (i.e., total hog inventory/km2; [27]), and presence of feral swine [28] into five regions; Alaska and Hawaii were included as their own, separate, regions. The seven US regions used in this risk assessment are: Northeast (Region 1: Maine, Massachusetts, New Hampshire, New York, and Vermont); Midwest (Region 2: Connecticut, Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, North Dakota, Nebraska, New Jersey, Ohio, Pennsylvania, Rhode Island, South Dakota, West Virginia, and Wisconsin); South (Region 3: Alabama, Arkansas, Delaware, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, and Virginia); Rocky Mountain (Region 4: Arizona, Colorado, Montana, Nevada, New Mexico, Utah, and Wyoming); West (Region 5: California, Washington, Oregon, and Idaho), Alaska (Region 6), and Hawaii (Region 7).
Fig. 1.
A map of the airports (red bubbles) and coastal and inland (blue bubbles) ports that received at least one aircraft/ship from the region of distribution during 2017–2021 or 2017–2020, respectively. The size of the bubble represents the average number of aircrafts/ships received at each port per year during the respective time frame, scaled as the minimum average number of crafts (n = 0.2) is equal to 1 and the maximum (n = 3918) is equal to 1000. The regions at risk (n = 7) considered in the risk assessment are outlined and delineated by color. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3. Rate of introduction
The rate of introduction is calculated based on entry (i.e., the number of infected entries per pathway), transmission (i.e., the basic reproduction number (R0) of the virus), and establishment (i.e., the successful first transmission of the virus to a local host/vector and continued transmission between local hosts and vectors). For this step, the region of distribution (i.e., JEV endemic and epidemic areas in Southeast Asia and the Western Pacific rim, including Australia) was treated as a single region.
2.3.1. Entry
The annual rate of entry is calculated considering the annual volume of animals, vectors or commodities moved along each pathway from the region of distribution to each region at risk, the probability that the pathogen (i.e., JEV) is viable in the host or vector upon arrival in the region at risk, and the probability of persistence of infection in the host or vector despite control measures applied to prevent the entry of the pathogen (e.g., application of insecticides, quarantine, etc.).
All of the pathways assessed in the previous risk assessment were re-evaluated using the FEVER framework. The following pathways were evaluated: entry via infected mosquito eggs/larvae in imported goods, infected adult mosquitoes via active or passive flight, infected adult mosquitoes via aircraft, ships, or shipping containers, infected humans, legal and illegal trade of infected livestock, infected rodents, infected exotic/zoo animals, infected migrating ardeid birds, legal and illegal trade of animal products, genetic and biological materials, and vaccines. The only difference in the assessed pathways was the introduction of infected mosquito eggs/larvae in imported goods which was evaluated using two separate pathways: eggs/larvae via imported used tires and eggs/larvae via imported plants. Whereas these are not the only commodities imported into the US that may harbor eggs/larvae of mosquitoes that can transmit JEV, they are two known pathways for the introduction of non-native mosquitoes [29,30]. Since these are the two best quantified pathways of egg/larvae introduction, despite there being limited information for both, we chose these pathways to serve as a proxy/estimation for egg/larvae introduction through imported goods.
If a pathway was determined to have negligible risk for virus entry from the region of distribution in the previous risk assessment and remained so after the re-evaluation, it was not included in this risk assessment. The pathways that were not evaluated in the semi-quantitative risk assessment included: infected adult mosquitoes via active or passive flight, infected humans, legal and illegal trade of infected livestock, infected rodents, infected exotic/zoo animals, legal and illegal trade of animal products, genetic and biological materials, and vaccines (Table 2).
Table 2.
The pathways of entry determined negligible by a FEVER assessment and the accompanying rationale.
| Pathway | Rationale |
|---|---|
| Infected vector | |
| Adult mosquitoes via active or passive flight | No new information from the previous FEVER assessment. The distance from the Asian and Australian continent is too far for active or passive flight [18]. |
| Infected host | |
| Human migrants or tourists | Humans are considered dead-end hosts as they do not produce enough viremia to infect mosquitoes [106]. There is not enough information available to consider a zoonotic vector-free transmission route (i.e., aerosol transmission). |
| Livestock trade - Legal | The importation of live swine is currently prohibited from all countries in the region of distribution [107]. At the time of assessment (2023), live poultry can only be imported from or transit through Australia, Brunei, Japan, Singapore, Sri Lanka, Papua New Guinea, and Timor-Leste. However, the required quarantine period of 30 days would preclude any viremic period in birds (∼14 days [87]) [108]. |
| Livestock trade - Illegal | There is no reliable information on the number of swine and poultry illegally imported into the US from the region of distribution. Given the size of the animals and proximity of the countries, it is assumed the number moved in fewer than 14 days (the outer limit for birds [87]) (i.e., via airplane) and not intercepted is negligible. |
| Rodents | While there is evidence of moderate JEV seroprevalence in rodents (45%) within the region of distribution (China) [109], the time required for ship travel from the region of distribution to the US would preclude the viremic period (3–4 days; [110,111]) and the additional regulations around inspections and pest control would reduce introduction via this pathway to negligible. |
| Legal movement of exotic/zoo animals | The main species of concern are birds and swine. Information on the exact number imported is not available, it is assumed very few are being imported from the region of distribution. In addition, the regulations/quarantine measures (minimum 30 days) in effect would preclude the viremic phase [112,113]. |
| Animal products – Legal and illegal trade | There is no evidence that animal products can transmit JEV. |
| Genetic (i.e., semen, embryo, ova) and biological (i.e., serum) materials | The importation of swine products into the US from countries in the region of distribution is prohibited [107]. |
| Other | |
| Vaccines | There are no vaccines approved for animal use in the US. The only licensed and available human vaccine (IXIARO®; Valneva Austria GmbH) is an inactivated vaccine and manufactured in Livingston, United Kingdom, outside the region of distribution [114]. |
Countries included in the region of distribution: Australia, Bangladesh, Bhutan, Brunei, Cambodia, China, India, Indonesia, Japan, Lao People's Democratic Republic, Malaysia, Myanmar, Nepal, North Korea, Pakistan, Papua New Guinea, Philippines, Russia, Singapore, South Korea, Sri Lanka, Taiwan, Thailand, Timor-Leste, Vietnam.
The following pathways were evaluated in the semi-quantitative risk assessment: entry via infected mosquito eggs/larvae in imported tires, or imported plants, adult mosquitoes via aircraft, ships, or shipping containers, and infected migrating ardeid birds. The calculation of the number of vectors introduced via imported tires, imported plants, aircraft, ships, and shipping containers was done similarly. Details for each pathway are described below, but in brief, the number of commodities/vessels coming from the region of distribution to each US region was estimated and then multiplied by the estimated number of vectors introduced per commodity/vessel. This number was then transformed into its appropriate qualitative category for use in MINTRISK (Fig. S2).
To calculate the number of mosquito eggs and larvae transported by imported tires, first the number of imported used pneumatic tires from the region of distribution into each US region was estimated by multiplying the total number of imported used pneumatic tires in 2020 (HS Code 401220; [31]) by the weighted regional expenditure for imported rubber products in 2020 (HS Code 40; [32]). Then, the estimated number of tires was multiplied by the average proportion of mosquito positive tires out of the number sampled, and this number was multiplied by the average number of mosquito eggs and larvae per tire to estimate the input parameter for the annual number of eggs and larvae. The proportion of tires with invasive mosquitoes and the number of mosquito eggs and larvae per tire were calculated for each container searched (n = 3) described in Laird et al. [33] and yearly (2010–2016) in Ibáñez-Justicia et al. [34]. Independently for each paper, the average proportion of positive tires and the number of mosquito eggs and larvae overall in all container searches (0.010 of tires searched were positive, and the positive tires had an average of 2.75 vectors/tire; [33]) and years (0.019 of tires searched were positive, and the positive tires had an average of 1.91 vectors/tire; [34]) was calculated. To estimate the uncertainty around the input parameter, the minimum and maximum values across all container searches/years were identified and used to calculate the annual number of eggs and larvae, if the qualitative category of the minimum and/or maximum was the same as the average it was designated as “low”, one category lower/higher, “moderate”, and more than a two-category difference was “high”.
The corresponding qualitative values for the average, minimum, and maximum were compared between studies. There were no differences in the qualitative categories determined for each region at risk between the two calculations for the average and maximum annual number of vectors, however, the estimates for the minimum annual number of vectors from Laird et al. [33] were one category lower or the same as those from Ibáñez-Justicia et al. [34] and thus were chosen to represent the minimum categories as it would encompass the most uncertainty in this parameter.
The number of mosquito eggs and larvae transported by imported plants was calculated similarly to imported used tires; the total number of imported plants (i.e., live bulbs, tubers, tuberous roots, corms, crowns and rhizomes, dormant or in growth/flower, live edible fruit or nut trees, shrubs, and bushes, and live unrooted cuttings and slips) in 2020 (HS Codes 60110, 60120, 60210, and 60220; [[35], [36], [37], [38]]) was multiplied by the weighted regional expenditure for imported plant products (HS Code 06; [32]) to estimate the total number of imported products per region at risk. The United Nations Trade Commodity Statistics varies in its reporting of imported plants, either quantity (i.e., integer value) and/or kilograms are reported, and only the exact number (i.e., quantity) was utilized in this calculation. This number was then multiplied by the average number of mosquito eggs and larvae collected in plants (i.e., “Lucky Bamboo”). Two studies were considered to evaluate the number of mosquito eggs and larvae, Demeulemeester et al. [39] and Ibáñez-Justicia et al. [34]. Given the methods of the two studies, the average from Demeulemeester et al. (0.003; [39]), was considered at the upper end of the estimation of the number of vectors per plant, as they were actively searching for eggs/larvae, whereas Ibáñez-Justicia et al. (0.00066; [34]) was considered at the lower end as they only actively searched for larvae if an invasive adult mosquito species was sampled using a BG-Sentinel and a CO2 baited Mosquito Magnet Liberty Plus traps at the specific timepoint. Both values were used to calculate the number of vectors and the qualitative categories corresponding to the values from the maximum estimation were utilized as the input parameter. To assess the uncertainty, if the qualitative categories corresponding to the calculated values from the two estimations were different, the uncertainty was “high”, but if the result was within the same qualitative category the uncertainty was considered “moderate”. For five of the seven regions at risk, the lower estimation of the number of mosquito eggs/larvae was only one qualitative category lower than the higher estimation.
To calculate the number of adult mosquitoes transported by airplanes, the average number of non-stop flights from the region of distribution to a port within the region at risk from 2017 through 2021 [40] (Fig. 1) was multiplied by the average number of vectors collected in airplanes (0.13; from [[41], [42], [43], [44], [45]]). To assess the uncertainty, the average number of flights was multiplied by study with the least vectors sampled per plane (0.04/plane; [41]) and the most (2.2/plane; [42]) to indicate whether the uncertainty should be low (no difference in category between the extremes and the average), moderate (either one or both extremes had a difference of one category different), or high (either one or both extremes had a difference of more than one category).
To calculate the number of adult mosquitoes transported by ships, the average yearly number of ship voyages with an origin port in the region of distribution to a port within the region at risk from 2017 to 2020 [46] (Fig. 1) was multiplied by the proportion of inspected ships found positive for presence of mosquitoes (0.5681), to account for ships without mosquitoes, and the adjusted number was multiplied by the average number of mosquitoes collected in ships (19.09) from Nie et al. [47]. To calculate the number of adult mosquitoes introduced via shipping containers by ship, the number of containers was estimated from the average yearly tonnage with an origin port in the region of distribution shipped to a port within the region at risk from 2017 to 2020 [46]. Based on the United States Army Corps of Engineers [48], the average percentage of foreign-borne US waterborne commerce by weight that was containerized from 2017 to 2020 was 21.5% (range 21.0% to 22.4%); to estimate the amount of cargo that was containerized, the average yearly tonnage was multiplied by 0.215. To estimate the number of containers, the average yearly tonnage adjusted for the amount containerize was divided by 26.28 tons (i.e., the average tonnage of a fully loaded twenty-foot container (TEU) (from [49])). This number was then multiplied by the number of vectors collected in containers (0.000355; [50] cited by [17]).
To evaluate the pathway of infected migratory ardeid birds, the ardeid species found in the US were identified through Birds of the World [51]. Twenty-one species were identified and their individual species pages (see Table S3) were searched for information on distribution and migration patterns. In addition, the “Wading birds” section of Rare Birds of North America [52] was searched. If a species was known to have movement through a country in the region of distribution and a sighting in a region at risk, the number of sightings was used to estimate the number of migrating ardeid birds into the respective region at risk.
2.3.2. Transmission
There is no information on the basic reproduction ratio (R0) of JEV in the US, as JEV is not found in the US, therefore we utilized information from transmission models for JEV in endemic countries and for other diseases in the US. To estimate the overall R0 for JEV, we incorporated information from two transmission cycles: domestic pig – mosquito – domestic pig and wild bird – mosquito – wild bird. The basic reproduction ratio (i.e., the number of secondary infected pigs (domestic) in a completely susceptible population from a single infected pig (domestic) via mosquito) ranged from 1.07 to 2.48 from transmission models in the endemic areas of Bangladesh and Cambodia [[53], [54], [55]]. As an approximation for the R0 for JEV from the wild bird – mosquito – wild bird cycle, the R0 for WNV for different regions of the contiguous US (R0 = 0.94–2.42; [56]) was utilized. As WNV is not found in Hawaii, avian malaria was used as a guide to estimate the R0 in this region (R0 = 1.74–6.09; [57]). As the estimated R0 for WNV and JEV mostly fell in the moderate category, all regions were deemed to have a moderate R0, except for Alaska, and the uncertainty around that value increased from low to moderate if the region was known to have a large swine (domestic and/or feral) population. Although the R0 for avian malaria ranged from moderate to high in Hawaii, we chose to model transmission as moderate, as the study gave indication that a mosquito-wild bird-mosquito transmission cycle is viable, however given the discrepancies in the pathogen and host systems, we chose to remain conservative with the assumption that transmission in Hawaii would not be vastly different to areas of the contiguous US. As in Hawaii, WNV is not found in Alaska either, however given the absence of feral pigs [28,58], low domestic pig population [27], few ardeid species with low relative abundance [59], and a short vector window, Alaska was deemed to have a low R0 with a moderate uncertainty.
2.3.3. Establishment
Establishment is considered in two steps. For the first transmission step of establishment, given a specific pathway, region at risk and time of entry, the introduced infected vector or host must find a susceptible local host or competent local vector, respectively. Assuming the first step has occurred, for the second step of establishment to occur, either a locally infected host or vector transmits JEV to a competent local vector or local susceptible host, respectively.
2.3.3.1. First transmission step
For the first transmission step of establishment, several criteria were categorized independently for each question and then combined as a multiplicative probability where the result could not be higher than the lowest probability. The three criteria incorporated for pathways of mosquito vector introduction were survival to adulthood and biting, host overlap at the port of entry, and transmission rate for the vector species of interest. The two criteria incorporated for the pathway of migratory ardeids were the overlap of the sightings of the migrant birds, based on [52], with the mosquito season for the region and the infection rate of putative US vectors from [25] and an update of this meta-analysis.
As the pathways for mosquito introduction were focused on different genera, for the first criterion, the introduction pathways of mosquito eggs and larvae via used tires and plants included hatching success of Zika infected Aedes albopictus under a daily fluctuating temperature [60], which was considered low, and then modified by the probability of survivability based on the persistence suitability predicted for Ae. albopictus based on temperature in each region [61]. For the introduction of adult mosquitoes via ship, container, and aircraft, the first criterion of the probability of survival was based on the length of travel time and experimental Culex spp. (exclusive to Cx. pipiens, quinquefasciatus, and restuans species) survival times at multiple temperatures [62].
The second criterion was evaluated similarly for all pathways. To estimate the probability of an introduced infected mosquito infecting a local host (i.e., feral swine, domesticated swine, or ardeid birds) with JEV, we considered the number of host sightings within the maximum active flight distance for Culex (7.7 km; [63]) from seaports and airports of entry and the density (pig/km2) of domestic swine in the ports' home county. The location of airports with at least one non-stop flight from the region of distribution in 2017–2022 was identified from the Bureau of Transportation Statistics (BTS) Master Coordinate File [64] and the location of coastal and inland ports with at least one ship from the region of distribution in 2017–2020 was identified from the BTS Principal Port list [65]. If the coastal/inland port could not be identified by the list, the location was estimated as the nearest city. To calculate the number of host sightings near ports of entry, first, the locations of US feral pig observations in 2022 from iNaturalist [66] and the locations of Ardeidae bird observations in the US in 2022 from the eBird Observation Dataset [67] were downloaded from the Global Biodiversity Information Facility. Using the R language [68], we then counted the number of host observations within 7.7 km of each port of introduction's location [[69], [70], [71]]. To account for the uncertainty around the feral pig sightings, we included a 600-m buffer around each feral pig observation which corresponds to a core area of 1.09 km2 [72]. To calculate the density of domestic swine in each US county, the number of total hog inventory at the end of December in 2017 per county [27] was divided by the total land area (km2) of each county from the 2021 US Census Bureau (tigris package; [73]).
For the third criterion, the transmission rate of Aedes spp. were used for the mosquito eggs and larvae pathways, and of Culex spp. for the adult mosquitoes pathway, based on [25] and an update of this meta-analysis.
2.3.3.2. Second transmission step
For the migratory bird pathway three criteria were considered, similarly to the first transmission step; overlap of the date of the sightings of the migrant birds [52] with the mosquito season for the region, the median mean relative abundance of all ardeid species in the region [59], and the transmission rate of putative US vectors from [25] and an update of this meta-analysis. For the vector introduction pathways, the second transmission step of establishment was determined by expert opinion accounting for information on mosquito density and the presence of mosquito habitats at ports of entry.
2.4. Epidemic size
Epidemic size corresponds to the number of host animals infected after the disease is introduced, and is calculated utilizing the extent of spread in the first vector season and the probability of the virus to persist to the next vector season, or persistence. The risk score for spread is primarily based on the optimal reproduction number, the number of infection generations in one vector season, and the number of infection generations until detection of disease. Based on expert opinion, the number of infection generations per vector season (see IGseason in Table 1 in [20]) had substantial variation within regions. To account for this variation, we evaluated risk assessment models for both the minimum and maximum infection generation values, as determined by expert opinion (Table S4), for each region at risk.
3. Results
The median values and 95% uncertainty intervals (UI) for the risk scores for annual rate of entry, the optimal reproduction number (transmission), the probability of establishment, and the overall rate of introduction for all pathway and region combinations are reported in Supplementary Table 5 (Table S5). In Table 3, the median values and 95% UI for the risk scores for the annual extent of spread, likelihood of viral persistence overwintering, estimated epidemic size, economic impact, and overall risk estimate for regions with a non-negligible rate of introduction are reported.
Table 3.
Median value and 95% uncertainty interval (UI) of risk scores, along with the qualitative value for the median score, for the annual extent of spread, viral persistence overwintering, estimated epidemic size, economic impact, and overall risk into the South region for high and low estimates of vector infection generation times.
| Parameter | Region at risk |
||
|---|---|---|---|
| South (Region 3) | |||
| Median score (95% UI) |
QV | ||
| IGT = 10 | IGT = 15 | ||
| Annual extent of spread | 0.40 (0.05–1.25) |
0.56 (0.05–1.53) |
Moderate |
| Persistence | 0.99 (0.08–1.94) |
1.15 (−0.26–2.23) |
Very high |
| Epidemic size | 1.29 (0.46–1.38) |
1.30 (0.68–1.38) |
Very high |
| Economic impact | 1.21 (0.47–1.68) |
1.24 (0.58–1.68) |
Very high |
| Overall risk | 0.89 (0.11–1.41) |
0.92 (0.28–1.45) |
Very high |
IGT = Number of infection generations for one vector season; QV = qualitative value.
3.1. Entry
Based on the results of the previous FEVER assessment [18] and the current assessment, the following pathways were deemed of negligible risk and therefore not evaluated further: entry of infected adult mosquitoes via active or passive flight, infected humans, legal and illegal trade of infected livestock, infected rodents, infected exotic/zoo animals, legal and illegal trade of infected animal products, genetic and biological materials, and vaccines (Table 2). The pathways of entry evaluated in this risk assessment were entry via infected vector eggs/larvae in imported goods, infected adult vectors via aircraft, ships, or shipping containers, or infected ardeid birds migrating from the current region of distribution.
The median risk scores for annual rate of entry were above zero for all pathway by region combinations, except for adult mosquitoes via shipping container into the Northeast and Midwest regions and mosquito eggs and larvae via plants into Hawaii (Table S5, Fig. S3). The pathway with the highest median risk score for annual rate of entry into each region was: mosquito eggs and larvae via plants into the Northeast (median risk score = low), mosquito eggs and larvae via tires into the Midwest and South (median risk score = moderate and high, respectively), adult mosquito via ships into the West (median risk score = moderate), and adult mosquitoes via aircraft into the Rocky Mountain region, Alaska, and Hawaii (median risk score = low) (Table S5, Fig. S3).
3.2. Establishment
The probability of establishment was negligible in the Rocky Mountain region and Alaska for all pathways, but ranged from very low to moderate in the Northeast, negligible to moderate in the Midwest, very low to high in the South and Hawaii, and negligible to low in the West (Table S5). The highest probability of JEV establishment was in the South and Hawaii via infected migratory ardeids (0.62; 95% UI = 0.17–0.92); overall for this pathway the probability ranged from negligible in Rocky Mountain, West, and Alaska, moderate in the Northeast and Midwest, and high in the South and Hawaii regions. The probability of establishment via the introduction of mosquito eggs and larvae in tires and plants ranged from very low (Northeast, Midwest, and Hawaii) to low (South and West), adult mosquitoes via aircraft ranged from very low (Midwest, West, Hawaii) to low (Northeast, South), and adult mosquitoes via ships and containers ranged from negligible (Midwest) to low (West) for (Table S5).
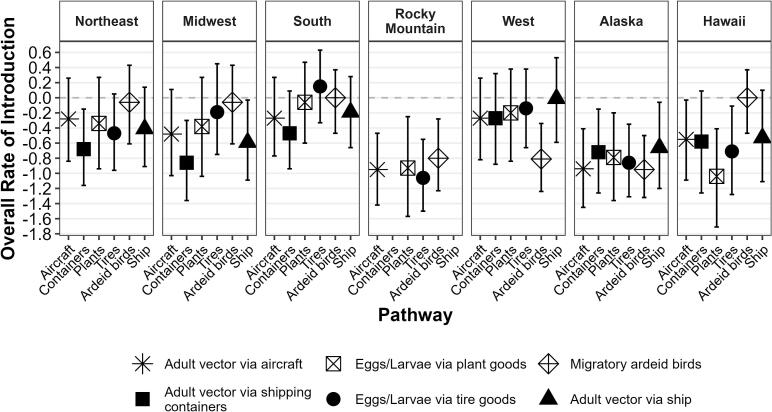
3.3. Rate of introduction
The median risk score for the overall rate of JEV introduction was equal to or below zero for all evaluated pathways in the Northeast, Midwest, Rocky Mountain, and West regions, and Alaska and Hawaii (Fig. 2); these regions were not evaluated further. The two pathways at zero were migrating ardeids into the South and Hawaii (median risk score = 0.00; 95% UI = −0.47–0.37; Table S5). For the South region, the three pathways with the highest median risk score were utilized in the subsequent model. These pathways were mosquito eggs and larvae via imported tires (median risk score = 0.15; 95% UI = −0.33–0.63), migrating ardeids (median risk score = 0.00; 95% UI = −0.47–0.37), and mosquito eggs and larvae via imported plants (median risk score = −0.06; 95% UI = −0.60–0.47).
Fig. 2.
Median value and 95% uncertainty interval of risk scores for the overall rate of introduction of Japanese encephalitis virus by pathway into seven US regions at risk.
3.4. Annual extent of spread, persistence, and estimated epidemic size
In the South region, the number of infected host animals at the end of the first vector season was moderate and the likelihood of persistence, or overwintering, of the virus was very high for both low and high infection generation times (Table 3). The higher risk in the South is largely a result of the very high likelihood of adult vectors surviving through winter, given warmer temperatures, until the next vector season and a larger number of infected hosts at the end of the first vector season (Table S3c). The estimated epidemic size was very high in the South (Table 3).
3.5. Overall risk
Summarizing the overall risk, the South region had a very high overall median risk score and very low/low to very high uncertainty interval (Table 3). The overall risk reflects the rate of introduction and economic impact of a JEV incursion into the region at risk. Exploring the components of the rate of introduction and economic impact (Fig. 3), in the South region, the highest median overall rate of introduction risk score was very low for infected mosquito eggs and larvae introduced via tires, with an uncertainty interval from negligible to high, and if introduced, the economic impact would be very high with an uncertainty interval of moderate to very high.
Fig. 3.
A probability-impact diagram illustrating the contribution of the rate of introduction and economic impact on the overall risk estimate of the South region. The median value for the risk score and error bars for the 95% uncertainty interval are displayed. Values outside the dashed grey box indicate extremely low (< 0) or extremely high (> 1) risk.
3.6. Socio-ethical and environmental impact
Socio-ethical impact considers the impact of the human disease burden and consequences for animal welfare, among other factors. The environmental impact includes the effect of the loss of biodiversity, nature values, and of insecticide use (Table S4). Due to the lack of information, the results of the socio-ethical and environmental impact were considered for the US as a single region. The socio-ethical and environmental impacts were very high and high with median risk scores of 0.80 (95% UI = 0.48–0.99) and 0.77 (95% UI = 0.50–0.98), respectively.
4. Discussion
This update of our group's previous qualitative risk assessment of a JEV incursion into the US [18] provides a more granular view of the different regions at risk within the US, now including Alaska and Hawaii, and incorporates other aspects from introduction to spread, for which there is limited data. Although exploring and comparing which area within the region of distribution would pose the highest risk is also possible, we chose to model and compare US regions at risk to determine which regions are at the highest risk of introduction, subsequent spread and persistence, and the associated impacts, as this information can directly guide preparedness and surveillance efforts in the US. We used a semi-quantitative risk assessment tool to compare and prioritize regions at risk for JEV incursion, rather than explicitly estimate the rate of introduction [20]. From our results, the South region should be prioritized for surveillance activities to enable early detection of JEV, given the results of a non-negligible rate of introduction. Additionally, preparedness strategies should be developed due to the large swine industry in this region.
Comparing the pathways' risk scores for the rate of entry between the previous and current risk assessments, here, we found that the risk score for the annual rate of entry of infected adult mosquitoes via ships ranged from low to moderate and was negligible to low via containers, except into the Rocky Mountain region where no ships or shipping containers were recorded. The rate of introduction of infected adult mosquitoes via ships and shipping containers was deemed negligible, similarly to the previous assessment. In this risk assessment, the risk score for the annual rate of entry of infected adult mosquitoes via aircraft was low, which, based on the corresponding semi-quantitative values (1–10 mosquitoes), this aligns with the results of Oliveira and colleagues' quantitative risk assessment of the number of infected mosquitoes introduced into the US via aircraft (1–7 mosquitoes) [17]. In this assessment, the overall rate of introduction for adult mosquitoes via aircraft was negligible; a product of the low annual rate of entry and negligible to low probability of establishment in areas surrounding airports.
A prominent difference between the two risk assessments concerned the entry of infected eggs/larvae via goods pathway. As discussed previously, we chose to use tire and plant goods as a proxy for all imported goods. The predominant species transported globally through these specific pathways are Aedes spp. [30], but Culex spp. and Ochlerotatus notoscriptus have been intercepted in cargo and used tires arriving via ship into New Zealand [74]. Our previous risk assessment focused on Culex species specifically, but Aedes species merited inclusion in this risk assessment as they have historically established in novel areas via trade of used tires, plants, and aircraft [[75], [76], [77]], they have moderate levels of JEV infection prevalence in the region of distribution [24], are mammophilic feeders [78,79], and a competent vector of JEV (i.e., Aedes japonicus, Aedes albopictus [25] and citations within [6]). Based on the lack of surveillance of mosquitoes transported in plant goods into the US, similar studies performed in other countries were used. However, these studies focused on “Lucky Bamboo”, a known risk commodity, which is likely to overestimate the number of eggs/larvae introduced as we included other types of plant goods in the calculation. Conversely, due to the calculation, we only utilized the exact quantity of plants imported, rather than using quantity plus weight, which would underestimate the amount of plant goods imported and likely underestimate the number of vectors from this pathway. For goods, interstate travel was not considered, and it was assumed they were delivered to their destination (i.e., region at risk). Although the inclusion of interstate travel would increase the number of vectors into new regions, the additional travel time would likely negate this increase in vector numbers as the likelihood of mosquito larval and hatched adult survival and transmission capability at the time of arrival would be reduced. However, the increase in travel time would likely not affect egg viability in the same way.
The rate of introduction is dependent on the pathways of entry and many assumptions were made due to the limited amount of information on mosquito movement via global travel and trade. To estimate the number of mosquitoes introduced, first, the annual number of aircraft, ships, shipping containers, or goods from the region of distribution to each region at risk was estimated, then, this number was scaled to the number of vectors introduced via those mechanisms. The number of ships and aircraft were likely to be underestimated, as we did not include military crafts, cruise ships, and personal crafts outside of our databases, and we limited the ships/flights to those with non-stop voyages from a port of origin in the region of distribution. To scale the number of aircraft, ships, containers, or goods to the number of vectors introduced, static summaries of the number of mosquitoes collected per craft/container/good were used as there is limited surveillance information on the number of mosquitoes imported by ships, aircraft, and goods, including larvae and adult mosquito trapping in the port vicinity. In addition, specifics, such as type of aircraft/vessel or the clustered nature of infestation, were not addressed [19]. Although this approach was adequate to achieve our objective of prioritization of US regions at highest risk for JEV introduction, further investigation to quantify the number of infected mosquitoes introduced via goods would be valuable.
In addition, as this was an update of a previous risk assessment, we limited the time frame of data to include 2017 to 2022, the year the current assessment began. Although data were available for aircraft voyages for all years, data on ship voyages was only available for 2017 to 2020. Notable during this period was the dramatic decrease in global travel and trade as a result of restrictions enacted after the declaration of the COVID-19 Pandemic in March 2020 and the slow revival in subsequent months. This time frame was chosen as we wanted to reflect the current risk for incursion using the most up-to-date information possible, however the choice of this time frame parameterized the risk assessment with a period of lower air travel [80] and maritime trade movement [81] than what was seen in the previous years and is likely to underestimate the rate of introduction of an infected adult mosquito via aircraft or ship as travel increases. Similarly, the period (i.e., 2020) used to estimate the number of goods imported from the region of distribution was chosen as it was the most recent data available and very likely an underestimation of the number of imported goods previous to the COVID-19 Pandemic and ongoing recovery [80]. However, as changes to the supply chains, trade negotiations, and global travel are constantly occurring, risk assessments of JEV introduction, or other foreign animal diseases, should use current data and be updated as appropriate.
When comparing transmission and establishment between risk assessments, the transmission risk ranged from low to moderate, which is less variable than estimated in the previous risk assessment (low to high). While we maintained many of the original assumptions, a key difference was the inclusion of the basic reproduction number (R0) as a parameter in this risk assessment. To estimate this parameter, we utilized information on the R0 of WNV in the contiguous US [56] and avian malaria in Hawaii [57], as WNV is not found in Hawaii, to approximate the R0 of the wild bird – mosquito – wild bird transmission cycle. Although WNV is closely related to JEV, and it is assumed that there would be many similarities in transmission, there is no information on how similar their viral transmission would be. However, this assumption is the best current proxy for the R0 of JEV in the US, which is critical to our risk assessment model's calculation of the rate of introduction and epidemic size, underscoring the need to fully consider the uncertainty of the estimates when interpreting these results.
To approximate the pig – mosquito – pig transmission cycle, we incorporated the R0 from JEV transmission models parameterized with information from endemic countries, specifically South East Asia. Given the limited information about the R0 for JEV in general, information from these models was incorporated despite the limited applicability to US regions and swine production systems. As the primary production system in JEV endemic areas is subsistence backyard farming, these transmission models may be more applicable to areas with small farms and outdoor pork producers within the US. How the inclusion of high-density production systems would change local transmission in the US is unknown and transmission models that include these systems are necessary. In addition, the transmission models were parameterized based on information pertaining to JEV genotypes specific to these areas in South East Asia, predominantly GI and GIII [82], and how host and vector transmission rates would change based on a different genotype, for example GIV from Australia, was not evaluated. As we did not investigate which region was most likely to be a source of introduction and coupled with the lack of information on the fitness of different JEV genotypes in a novel system, our assessment of R0 and transmission should be interpreted as generic and not specific to any genotype.
Another assumption made was that ardeid and swine populations in the US would be naïve to a JEV infection. However, this is unlikely to be the case due to cross-protection with WNV [83] and possible protection of St. Louis encephalitis virus (SLEV) in birds [84] and longstanding antibody protection a previous infection with WNV may support in swine [85]. This cross-protection would likely reduce JEV transmission, but more information is needed to understand the impacts of cross-protection in host species on JEV transmission cycles and establishment.
Our determination of the probability of establishment was based, in part, on the availability of feral and domestic swine and ardeid bird host populations. Likewise to the ship, aircraft, and goods data, there were limitations on the recency and availability of data surrounding domestic and feral hogs, respectively. The domestic swine population in 2017 was used (73.14 million head on December 1st, 2017), as the most current census was not available, but is similar to the most current NASS numbers (75.46 million head; [86]). However, due to the lack of data on the numbers of feral swine in the US, observations in 2022 from a citizen science database (iNaturalist) was utilized as it included location information and was supplemented with broad estimates from the USDA national Feral Swine Damage Management program from 2014 to 2018 [58]. The numbers used in this assessment are likely an underestimate of the current number of feral swine in the US, particularly in the South and West regions, however it is unlikely to change the model estimates as host overlap (estimated as part of the first transmission step) was considered moderate/high to very high in these regions and the host population was estimated as very high in the South.
In addition, there are other hosts that were not accounted for as there is not enough information on their relative role in the JEV transmission cycle. There is evidence of other non-ardeid birds, commonly found in urban and industrialized areas, such as rock pigeons (Columba livia), house sparrows (Passer domesticus) and ring-billed gulls (Larus delawarensis), having moderate levels of viremia (i.e., 103.0–5.0 PFU/mL serum) after JEV inoculation in experimental settings [87]. More information about non-ardeid wild bird transmission effectiveness and their populations in and around ports is needed to determine their role in the JEV transmission cycle and impact on establishment in the US. In addition to wild birds, more information is also needed on the relative role of domestic birds in JEV transmission cycles [88,89]. Large flocks, such as in broiler or egg production, may have a large impact on transmission in US states without large swine populations (e.g., Georgia; [86,90]), or on a smaller scale, backyard chickens may pose a public health risk facilitating JEV transmission in more urban areas. Outside of the established transmission cycles including swine and ardeid birds, there is evidence that bats can transmit and overwinter with JEV, but there is little information about their role in the transmission cycle [91]. In addition to host presence and abundance, the establishment of a disease also depends on the presence and abundance of competent vectors in the region at risk. The risk of transmission and establishment is influenced not only by the proportion of infectious mosquitoes in a population but also by their overall abundance and biting preferences. Unfortunately, data on mosquito abundance and regional distribution are largely lacking, which hampers accurate risk assessment. An objective of this update was to provide a more granular assessment of the risk of JEV introduction into the US, by evaluating regions separately. Although the variation within each region was considered, the final risk scores assume a “homogenous” risk throughout the respective region, which is not reflective of the reality. Despite attempts to group similar states into regions, the US varies considerably in terms of climate, geography, livestock production, and biological diversity, even within a single state. While some regions display immense climate and land use variation, like the West and Midwest regions, establishment in the South is likely more homogenous, given the predictions of feral pig habitat [92] and sightings as well as climatological similarity throughout the region. One caveat to this hypothesis, is the very high density of swine production in North Carolina. As discussed previously, how areas of high-density pig production will affect local transmission is unknown and needs more research. Changes in climatic conditions, such as increased rainfall and temperature, can create new favorable niches for mosquitoes to thrive. Similarly, flooding or tropical storms can alter the timing and routes of bird migration, promoting the invasion and expansion of hosts into new areas. Urbanization and the intensification of agricultural practices, often through deforestation, push humans into areas previously inhabited by wildlife. More research is needed to fully grasp the intricate interplay between climate change and disease transmission [93].
The two components of a risk assessment are the likelihood of and the biological and economic consequences, or impacts, of the risk occurring [94]. In the current risk assessment, we determined that the South is at a higher risk of JEV incursion compared to other areas of the US. Regarding the consequences of a JEV incursion, the current risk assessment tool incorporates the economic impact to the US swine industry. Whereas JEV-associated reproductive and neurological illness in pigs is documented throughout the native range and in experimental studies [88], the economic impact to the US swine production system is unknown. The economic impact from our risk assessment considers the size of the host population and direct and indirect costs; here, we considered domestic and feral swine as well as ardeid birds in the host population. Similarly, for the South, where the large feral swine and ardeid population was included in the assessment, the economic impact is reflective of a worst-case scenario should an incursion occur in North Carolina, where a majority of the swine production occurs in this region.
In regard to the economic costs, we used information from the recent outbreak in Australia to provide estimates about the potential direct economic losses, but we were unable to determine the extent of all expected indirect costs incurred by a potential outbreak in the US as, at the time of writing of this manuscript, there is no final version available of a USDA-APHIS Foreign Animal Disease Preparedness and Response Plan (FAD PReP) document for JEV. The draft, currently under review, prescribes quarantine and stop movement control for swine in the control area, although it is unclear at what level, whether state or federal, and for how long. As demonstrated in other foreign animal disease outbreak exercises, the swine industry has limited capabilities to hold inventory, as such these response strategies could lead to additional economic impacts. As with all aspects of a risk assessment, the results are entirely dependent on the assumptions made, here it is important to note the reliance on the Australian experience and urge the consideration of regionalization and scale in the production systems between Australia and the US in subsequent agricultural economic assessments. In addition to the economic impact, socio-ethical and environmental impacts are integrated into the risk assessment tool, but due to the limited information available many of the questions were left as “Unknown”; a thorough assessment of these impacts is needed.
The following are the supplementary data related to this article.
Supplementary Fig. S1.
A map of the US delineating the regions at risk (n = 7) considered in the risk assessment of the introduction and impact of JEV incursion. Northeast (R1: Maine, Massachusetts, New Hampshire, New York, and Vermont); Midwest (R2: Connecticut, Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, North Dakota, Nebraska, New Jersey, Ohio, Pennsylvania, Rhode Island, South Dakota, West Virginia, and Wisconsin); South (R3: Alabama, Arkansas, Delaware, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, and Virginia); Rocky Mountains (R4: Arizona, Colorado, Montana, Nevada, New Mexico, Utah, and Wyoming); West (R5: California, Washington, Oregon, and Idaho), Alaska (R6), and Hawaii (R7).
Supplementary Fig. S2.
A chord diagram representing the amount of vectors/hosts moved along each pathway from the region of distribution to each region at risk (RAR). Mosquito eggs and larvae are moved via used tires and plant goods, whereas adult mosquitoes are moved via aircraft, ships, and shipping containers. The width of the arrows corresponds to the semi-quantitative value used in the MINTRISK model.
Supplementary Fig. S3.
Median value and uncertainty intervals for risk scores of annual rate of entry of Japanese encephalitis virus by pathway and region. Vectors transported along aircraft, shipping containers, and ships are adult mosquitoes and mosquito eggs and larvae are transported via plant goods and used tires. Migrating birds refer to infected ardeid hosts.
Parameter inputs for FEVER and MINTRISK to qualitatively assess the incursion risk of JEV for seven US regions.
Median value and 95% uncertainty interval (UI) of the risk score for the annual rate of entry, the probability of transmission, the probability of establishment, and overall rate of introduction of Japanese encephalitis virus by introduction pathway into seven US regions at risk.
Funding
This work was funded by the Swine Health Information Center (#22–072) with support from the Center for Outcomes Research and Epidemiology, and the College of Veterinary Medicine at Kansas State University.
CRediT authorship contribution statement
Andrea L. Dixon: Conceptualization, Data curation, Investigation, Software, Supervision, Visualization, Writing – original draft. Ana R.S. Oliveira: Conceptualization, Data curation, Investigation, Writing – review & editing. Lee W. Cohnstaedt: Data curation, Investigation, Writing – review & editing. Dana Mitzel: Writing – review & editing. Chad Mire: Funding acquisition, Writing – review & editing. Natalia Cernicchiaro: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all members of our advisory group as well as additional experts for their time and feedback when completing the surveys and request for data and expertise. Also, we would like to thank Dr. Christy Hanthorn for her insights and discussions on the topic and Ashley Thackrah for her help with data entry.
Data availability
The location of the publicly available datasets utilized in this study can be found cited in this article and supplemental material.
References
- 1.Campbell G.L., Hills S.L., Fischer M., Jacobson J.A., Hoke C.H., Hombach J.M., Marfin A.A., Solomon T., Tsai T.F., Tsu V.D., Ginsburg A.S. Estimated global incidence of Japanese encephalitis: a systematic review. Bull. World Health Organ. 2011;89(766–774) doi: 10.2471/BLT.10.085233. 774A-774E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Organisation for Animal Health Japanese Encephalitis. 2019. https://www.woah.org/app/uploads/2021/03/japanese-encephalitis.pdf
- 3.Le Flohic G., Porphyre V., Barbazan P., Gonzalez J.-P. Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricklin M.E., García-Nicolás O., Brechbühl D., Python S., Zumkehr B., Nougairede A., Charrel R.N., Posthaus H., Oevermann A., Summerfield A. Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat. Commun. 2016;7:10832. doi: 10.1038/ncomms10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai C., Palinski R., Xu Y., Wang Q., Cao S., Geng Y., Zhao Q., Wen Y., Huang X., Yan Q., Ma X., Wen X., Huang Y., Han X., Ma W., Wu R. Aerosol and contact transmission following intranasal infection of mice with japanese encephalitis virus. Viruses. 2019;11 doi: 10.3390/v11010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auerswald H., Maquart P.-O., Chevalier V., Boyer S. Mosquito vector competence for Japanese encephalitis virus. Viruses. 2021;13 doi: 10.3390/v13061154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon-Loriere E., Faye O., Prot M., Casademont I., Fall G., Fernandez-Garcia M.D., Diagne M.M., Kipela J.-M., Fall I.S., Holmes E.C., Sakuntabhai A., Sall A.A. Autochthonous Japanese encephalitis with yellow fever coinfection in Africa. N. Engl. J. Med. 2017;376:1483–1485. doi: 10.1056/NEJMc1701600. [DOI] [PubMed] [Google Scholar]
- 8.Platonov A., Rossi G., Karan L., Mironov K., Busani L., Rezza G. Does the Japanese encephalitis virus (JEV) represent a threat for human health in Europe? Detection of JEV RNA sequences in birds collected in Italy. Euro Surveill. 2012;17 doi: 10.2807/ese.17.32.20241-en. [DOI] [PubMed] [Google Scholar]
- 9.Preziuso S., Mari S., Mariotti F., Rossi G. Detection of Japanese encephalitis virus in bone marrow of healthy young wild birds collected in 1997-2000 in Central Italy. Zoonoses Public Health. 2018;65:798–804. doi: 10.1111/zph.12501. [DOI] [PubMed] [Google Scholar]
- 10.Ravanini P., Huhtamo E., Ilaria V., Crobu M.G., Nicosia A.M., Servino L., Rivasi F., Allegrini S., Miglio U., Magri A., Minisini R., Vapalahti O., Boldorini R. Japanese encephalitis virus RNA detected in Culex pipiens mosquitoes in Italy. Eurosurveillance. 2012;17 doi: 10.2807/ese.17.28.20221-en. [DOI] [PubMed] [Google Scholar]
- 11.Mackenzie J.S., Williams D.T., van den Hurk A.F., Smith D.W., Currie B.J. Japanese encephalitis virus: the emergence of genotype IV in Australia and its potential Endemicity. Viruses. 2022;14 doi: 10.3390/v14112480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams C.R., Webb C.E., Higgs S., van den Hurk A.F. Japanese encephalitis virus emergence in Australia: public health importance and implications for future surveillance. Vector-Borne Zoon. Diseases. 2022;22:529–534. doi: 10.1089/vbz.2022.0037. [DOI] [PubMed] [Google Scholar]
- 13.Brinkhoff M.N. Comment on van den Hurk et al. The Emergence of Japanese encephalitis virus in Australia in 2022: existing knowledge of mosquito vectors. Viruses. 2022;14:1208. doi: 10.3390/v15020270. Viruses 15 (2023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nett R.J., Campbell G.L., Reisen W.K. Potential for the emergence of Japanese encephalitis virus in California. Vector-Borne Zoon. Diseases. 2009;9:511–517. doi: 10.1089/vbz.2008.0052. [DOI] [PubMed] [Google Scholar]
- 15.van den Hurk A.F., Ritchie S.A., Mackenzie J.S. Ecology and geographical expansion of Japanese encephalitis virus. Annu. Rev. Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 16.Michaud K., Iverson G., Reiskind M.H., Kearney G., Richards S.L. Brief review of Japanese encephalitis virus: recommendations related to North Carolina swine farms and wider implications for swine farming. Parasitologia. 2022;2:302–312. doi: 10.3390/parasitologia2040025. [DOI] [Google Scholar]
- 17.Oliveira A.R.S., Piaggio J., Cohnstaedt L.W., McVey D.S., Cernicchiaro N. A quantitative risk assessment (QRA) of the risk of introduction of the Japanese encephalitis virus (JEV) in the United States via infected mosquitoes transported in aircraft and cargo ships. Prev. Vet. Med. 2018;160:1–9. doi: 10.1016/j.prevetmed.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira A.R.S., Piaggio J., Cohnstaedt L.W., McVey D.S., Cernicchiaro N. Introduction of the Japanese encephalitis virus (JEV) in the United States - a qualitative risk assessment. Transbound. Emerg. Dis. 2019;66:1558–1574. doi: 10.1111/tbed.13181. [DOI] [PubMed] [Google Scholar]
- 19.Horigan V., Simons R., Kavanagh K., Kelly L. A review of qualitative risk assessment in animal health: suggestions for best practice. Front. Veterin. Sci. 2023;10 doi: 10.3389/fvets.2023.1102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vos C.J., Hennen W.H.G.J., van Roermund H.J.W., Dhollander S., Fischer E.A.J., de Koeijer A.A. Assessing the introduction risk of vector-borne animal diseases for the Netherlands using MINTRISK: a model for INTegrated RISK assessment. PLoS One. 2021;16 doi: 10.1371/journal.pone.0259466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.More S., Bicout D., Bøtner A., Butterworth A., Calistri P., De Koeijer A., Depner K., Edwards S., Garin-Bastuji B., Good M., Gortazar Schmidt C., Michel V., Miranda M.A., Nielsen S.S., Raj M., Sihvonen L., Spoolder H., Thulke H.-H., Velarde A., Willeberg P., Winckler C., Bau A., Beltran-Beck B., Carnesecchi E., Casier P., Czwienczek E., Dhollander S., Georgiadis M., Gogin A., Pasinato L., Richardson J., Riolo F., Rossi G., Watts M., Lima E., Stegeman J.A. Vector-borne diseases. EFSA J. 2017;15 doi: 10.2903/j.efsa.2017.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen S.S., Alvarez J., Bicout D.J., Calistri P., Depner K., Drewe J.A., Garin-Bastuji B., Rojas J.L.G., Schmidt C.G., Michel V., Chueca M.Á.M., Roberts H.C., Sihvonen L.H., Stahl K., Calvo A.V., Viltrop A., Winckler C., Bett B., Cetre-Sossah C., Chevalier V., Devos C., Gubbins S., Monaco F., Sotiria-Eleni A., Broglia A., Abrahantes J.C., Dhollander S., Stede Y.V.D., Zancanaro G. Rift Valley fever - epidemiological update and risk of introduction into Europe. EFSA J. 2020;18 doi: 10.2903/j.efsa.2020.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.C.J. de Vos, M.R. Hoek, E.A.J. Fischer, A.A. de Koeijer, J. Bremmer, 2012. Risk assessment framework for emerging vector-borne livestock diseases, Report 11-CVI0168,CVI.
- 24.Oliveira A.R.S., Cohnstaedt L.W., Strathe E., Hernandez L.E., McVey D.S., Piaggio J., Cernicchiaro N. Meta-analyses of the proportion of Japanese encephalitis virus infection in vectors and vertebrate hosts. Parasit. Vectors. 2017;10 doi: 10.1186/s13071-017-2354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira A.R.S., Cohnstaedt L.W., Strathe E., Etcheverry L., Mcvey D.S., Piaggio J., Cernicchiaro N. Meta-analyses of Japanese encephalitis virus infection, dissemination, and transmission rates in vectors. Am. J. Trop. Med. Hyg. 2018;98:883–890. doi: 10.4269/ajtmh.17-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck H.E., Zimmermann N.E., McVicar T.R., Vergopolan N., Berg A., Wood E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Scientific Data. 2018;5 doi: 10.1038/sdata.2018.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United States Department of Agriculture – National Agricultural Statistics Service US census of agriculture: United States. Total hog inventory by county, (2019) 2017. https://quickstats.nass.usda.gov/
- 28.United States Department of Agriculture - Animal and Plant Inspection Service Map of Feral Swine Populations 2021 by County. 2021. https://www.aphis.usda.gov/aphis/ourfocus/wildlifedamage/operational-activities/feral-swine/sa-fs-history
- 29.Craven R.B., Eliason D.A., Francy D.B., Reiter P., Campos E.G., Jakob W.L., Smith G.C., Bozzi C.J., Moore C.G., Maupin G.O. Importation of Aedes albopictus and other exotic mosquito species into the United States in used tires from Asia. J. Am. Mosq. Control Assoc. 1988;4:138–142. [PubMed] [Google Scholar]
- 30.Swan T., Russell T.L., Staunton K.M., Field M.A., Ritchie S.A., Burkot T.R. A literature review of dispersal pathways of Aedes albopictus across different spatial scales: implications for vector surveillance. Parasit. Vectors. 2022;15:303. doi: 10.1186/s13071-022-05413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Integrated Trade Solution United Nations Commodity Trade Statistics: United States Rubber; Used Pneumatic Tyres Imports by Country 2020. 1962. https://wits.worldbank.org/trade/comtrade/en/country/USA/year/2020/tradeflow/Imports/partner/ALL/product/401220 (accessed September 4, 2023)
- 32.globalEDGE, Michigan State University - International Business Center State level imported traded goods at the 2-digit HS Code level from USA Trade Online Data. 2020. https://globaledge.msu.edu/global-insights/by/state (accessed December 6, 2022)
- 33.Laird L., Calder L., Thornton R.C., Syme R., Holder P.W., Mogi M. Japanese Aedes albopictus among four mosquito species reaching New Zealand in used tires. J. Am. Mosq. Control Assoc. 1994;10:14–23. [PubMed] [Google Scholar]
- 34.Ibáñez-Justicia A., Koenraadt C.J.M., Stroo A., van Lammeren R., Takken W. Risk-based and adaptive invasive mosquito surveillance at lucky bamboo and used Tire importers in the Netherlands. J. Am. Mosq. Control Assoc. 2020;36:89–98. doi: 10.2987/20-6914.1. [DOI] [PubMed] [Google Scholar]
- 35.World Integrated Trade Solution United Nations Commodity Trade Statistics: United States Plants, live; Bulbs, Tubers, Tuberous Roots, Corms, Crowns and Rhizomes, Dormant Imports by Country in 2020. 1962. https://wits.worldbank.org/trade/comtrade/en/country/USA/year/2020/tradeflow/Imports/partner/ALL/product/060110 (accessed January 25, 2024)
- 36.World Integrated Trade Solution United Nations Commodity Trade Statistics: United States Plants, Live; Unrooted Cuttings and Slips Imports by Country in 2020. 1962. https://wits.worldbank.org/trade/comtrade/en/country/USA/year/2020/tradeflow/Imports/partner/ALL/product/060210 (accessed January 25, 2024)
- 37.World Integrated Trade Solution United Nations Commodity Trade Statistics: United States Plants, Live; Bulbs, Tubers, Tuberous Roots, Corms, Crowns and Rhizomes, in Growth or in Flower, Chicory Plants and Roots Other than of Heading no. 1212 Imports by Country in 2020. 1962. https://wits.worldbank.org/trade/comtrade/en/country/USA/year/2020/tradeflow/Imports/partner/ALL/product/060120 (accessed January 25, 2024)
- 38.World Integrated Trade Solution United Nations Commodity Trade Statistics: United States Plants, live; Edible Fruit or Nut Trees, Shrubs and Bushes, Grafted or Not Imports by Country in 2020. 1962. https://wits.worldbank.org/trade/comtrade/en/country/USA/year/2020/tradeflow/Imports/partner/ALL/product/060220 (accessed January 25, 2024)
- 39.Demeulemeester J., Deblauwe I., de Witte J., Jansen F., Hendy A., Madder M. First interception of Aedes (Stegomyia) albopictus in lucky bamboo shipments in Belgium. J. Am. Mosq. Control Assoc. 2014;32:14–16. [Google Scholar]
- 40.United States Department of Transportation: Bureau of Transportation Statistics T-100 Market (all carriers) 1990. https://transtats.bts.gov/Tables.asp?QO_VQ=EEE&QO_anzr=Nv4%FDPn44vr4%FDf6n6v56vp5%FD%25FLS14z%FDHE%FDg4nssvp%25FM-%FDNyy%FDPn44vr45&QO_fu146_anzr=Nv4%FDPn44vr45 (accessed August 24, 2022)
- 41.Basio R.G., Prudencio P., Chanco I.E. Notes on the aerial transportation of mosquitoes and other insects at the Manila international airport, Philippine. Entomologist. 1970;1:407–408. [Google Scholar]
- 42.Russell R.C. In: Commerce and the Spread of Pests and Disease Vectors. Laird M., editor. Praeger; New York: 1984. Rajapaksa, N., Whelan, P.I., Langsford, W.A., mosquito and other insect introductions to Australia aboard international aircraft, and the monitoring of disinsection procedures; pp. 109–142. [Google Scholar]
- 43.Takahashi S. In: Commerce and the Spread of Pests and Disease Vectors. Laird M., editor. Praeger; New York: 1984. Survey on accidental introductions of insects entering Japan via aircraft; pp. 65–80. [Google Scholar]
- 44.Evans B.R., Joyce C.R., Porter J.E. Mosquitoes and other arthropods found in baggage compartments of international aircraft. Mosq. News. 1963;23:9–12. [Google Scholar]
- 45.Scholte E.-J., Ibañez-Justicia A., Stroo A., Zeeuw J., den Hartog W., Reusken C. Mosquito collections on incoming intercontinental flights at Schiphol international airport, the Netherlands, 2010-2011. J. Eur. Mosquito Cont. Assoc. 2014;32:17–21. [Google Scholar]
- 46.US Army Corps of Engineers: Waterborne Commerce Statistics Center Foreign Cargo Inbound and Outbound. 2018. https://publibrary.planusace.us/#/series/Waterborne%20Foreign%20Cargo (accessed August 25, 2022)
- 47.Nie W.-Z., Li J.-C., Li D.-X., Wang R.-J., Gratz N. Mosquitoes found aboard ships arriving at Qinhuangdao port, P.R. China. Med. Entomol. Zool. 2004;55:333–335. doi: 10.7601/mez.55.333. [DOI] [Google Scholar]
- 48.Navigation and Civil Works Decision Support Center . Information, United States Army Corps of Engineers; 2017. The U.S. Coastal and Inland Navigation System: Transportation Facts &.https://iwrlibrary.sec.usace.army.mil/searchResults?series=Fact%20Cards (accessed February 6, 2024) [Google Scholar]
- 49.Menon H. What is TEU in shipping - everything you wanted to know. Marine Insight. 2022 https://www.marineinsight.com/maritime-law/teu-in-shipping-everything-you-wanted-to-know/ (accessed January 18, 2024) [Google Scholar]
- 50.Prepared for MAF Biosecurity Authority, by the Border Management Group, Sea Container Review. MAF Discussion Paper No: 35. 2003. www.maf.govt.nz/biosecurity/border/papers/sea-container-review/sea-container-review.pdf
- 51.Billerman S.M., Keeney B.K., Rodewald P.G., Schulenberg T.S., editors. Birds of the World. Cornell Laboratory of Ornithology; Ithaca, NY, USA: 2022. https://birdsoftheworld.org/bow/home [Google Scholar]
- 52.Howell S.N.G., Lewington I., Russell Will. Princeton University Press; 2014. Rare Birds of North America. [Google Scholar]
- 53.Ladreyt H., Chevalier V., Durand B. Modelling Japanese encephalitis virus transmission dynamics and human exposure in a Cambodian rural multi-host system. PLoS Negl. Trop. Dis. 2022;16 doi: 10.1371/journal.pntd.0010572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diallo A.O.I., Chevalier V., Cappelle J., Duong V., Fontenille D., Duboz R. How much does direct transmission between pigs contribute to Japanese encephalitis virus circulation? A modelling approach in Cambodia. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan S.U., Salje H., Hannan A., Islam M.A., Bhuyan A.A.M., Islam M.A., Rahman M.Z., Nahar N., Hossain M.J., Luby S.P., Gurley E.S. Dynamics of Japanese encephalitis virus transmission among pigs in Northwest Bangladesh and the potential impact of pig vaccination. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J., Huang J., Beier J.C., Cantrell R.S., Cosner C., Fuller F., Zhang G., Ruan S. Modeling and control of local outbreaks of West Nile virus in the United States. Discrete Cont. Dyn. Syst. - B. 2016;21:2423–2449. doi: 10.3934/dcdsb.2016054. [DOI] [Google Scholar]
- 57.Dahlin K., Feng Z. Modeling the population impacts of avian malaria on Hawaiian honeycreepers: bifurcation analysis and implications for conservation. Math. Biosci. 2019;318 doi: 10.1016/j.mbs.2019.108268. [DOI] [PubMed] [Google Scholar]
- 58.United States Department of Agriculture, National Feral Swine Damage Management program. Five Year Report FY14-FY18, n.d.
- 59.Fink D., Auer T., Johnston A., Strimas-Mackey M., Ligocki S., Robinson O., Hochachka W., Jaromczyk L., Rodewald A., Wood C., Davies I., Spencer A. Cornell Lab of Ornithology; Ithaca, New York: 2022. eBird Status and Trends, Data Version: 2021; Released: 2022. [DOI] [Google Scholar]
- 60.Jian X., Jiang Y., Wang M., Jia N., Cai T., Xing D., Li C., Zhao T., Guo X., Wu J. Effects of constant temperature and daily fluctuating temperature on the transovarial transmission and life cycle of Aedes albopictus infected with Zika virus. Front. Microbiol. 2023;13 doi: 10.3389/fmicb.2022.1075362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brady O.J., Golding N., Pigott D.M., Kraemer M.U.G., Messina J.P., Reiner R.C., Jr., Scott T.W., Smith D.L., Gething P.W., Hay S.I. Global temperature constraints on Aedes aegypti and ae. Albopictus persistence and competence for dengue virus transmission. Parasit. Vectors. 2014;7:338. doi: 10.1186/1756-3305-7-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ciota A.T., Matacchiero A.C., Kilpatrick A.M., Kramer L.D. The effect of temperature on life history traits of Culex mosquitoes. J. Med. Entomol. 2014;51:55–62. doi: 10.1603/ME13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verdonschot P.F.M., Besse-Lototskaya A.A. Flight distance of mosquitoes (Culicidae): a metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica. 2014;45:69–79. doi: 10.1016/j.limno.2013.11.002. [DOI] [Google Scholar]
- 64.United States Department of Transportation - Bureau of Transportation Statistics, Aviation Support Tables: Master Coordinate, (n.d.). https://www.transtats.bts.gov/Fields.asp?gnoyr_VQ=FLL (accessed September 12, 2023).
- 65.United States Department of Transportation - Bureau of Transportation Statistics Principal Ports. 2023. https://geodata.bts.gov/datasets/usdot::principal-ports/about (accessed September 19, 2023)
- 66.iNaturalist Research-grade Observations Occurrence Download from GBIF.org. 2023. [DOI] [Google Scholar]
- 67.eBird Observation Dataset Occurrence Download from GBIF.org. 2023. [DOI] [Google Scholar]
- 68.R Core Team R: A Language and Environment for Statistical Computing. 2023. https://www.R-project.org
- 69.Pebesma E. Simple features for R: standardized support for spatial vector data. R J. 2018;10:439–446. doi: 10.32614/RJ-2018-009. [DOI] [Google Scholar]
- 70.Pebesma E., Bivand R. 2023. Spatial Data Science: With Applications in R, Chapman and Hall/CRC. [DOI] [Google Scholar]
- 71.Dixon A. Estimation of Host Density Around ports.md. 2024. https://github.com/andixonksuvet/JEV-US-Risk-Assessment
- 72.Gabor T.M., Hellgren E.C., Van Den Bussche R.A., Silvy N.J. Demography, sociospatial behaviour and genetics of feral pigs (Sus scrofa) in a semi-arid environment. J. Zool. 1999;247:311–322. doi: 10.1111/j.1469-7998.1999.tb00994.x. [DOI] [Google Scholar]
- 73.Walker K. tigris: Load Census TIGER/Line Shapefiles. R package version 2.1. 2024. https://CRAN.R-project.org/package=tigris
- 74.Derraik J.G.B. Exotic mosquitoes in New Zealand: a review of species intercepted, their pathways and ports of entry. Aust. N. Z. J. Public Health. 2004;28:433–444. doi: 10.1111/j.1467-842X.2004.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 75.Hawley W.A., Reiter P., Copeland R.S., Pumpuni C.B., Craig G.B. Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- 76.Linthicum K.J., Kramer V.L., Madon M.B., Fujioka K. =surveillance-control team, introduction and potential establishment of Aedes albopictus in California in 2001. J. Am. Mosq. Control Assoc. 2003;19:301–308. [PubMed] [Google Scholar]
- 77.Lühken R., Brattig N., Becker N. Introduction of invasive mosquito species into Europe and prospects for arbovirus transmission and vector control in an era of globalization. Infect. Dis. Poverty. 2023;12:109. doi: 10.1186/s40249-023-01167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Richards S.L., Ponnusamy L., Unnasch T.R., Hassan H.K., Apperson C.S. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of Central North Carolina. J. Med. Entomol. 2006;43:543–551. doi: 10.1603/0022-2585(2006)43[543:hpoaad]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Faraji A., Egizi A., Fonseca D.M., Unlu I., Crepeau T., Healy S.P., Gaugler R. Comparative host feeding patterns of the Asian Tiger mosquito, Aedes albopictus, in Urban and Suburban Northeastern USA and Implications for Disease Transmission. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dube K., Nhamo G., Chikodzi D. COVID-19 pandemic and prospects for recovery of the global aviation industry. J. Air Transp. Manag. 2021;92 doi: 10.1016/j.jairtraman.2021.102022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.March D., Metcalfe K., Tintoré J., Godley B.J. Tracking the global reduction of marine traffic during the COVID-19 pandemic. Nat. Commun. 2021;12:2415. doi: 10.1038/s41467-021-22423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schuh Amy J., Ward Melissa J., Leigh Brown Andrew J., Barrett Alan D.T. Dynamics of the emergence and establishment of a newly dominant genotype of Japanese encephalitis virus throughout Asia. J. Virol. 2014;88:4522–4532. doi: 10.1128/jvi.02686-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nemeth N.M., Bosco-Lauth A.M., Bowen R.A. Cross-protection between West Nile and Japanese encephalitis viruses in red-winged blackbirds (Agelaius phoeniceus) Avian Dis. 2009;53:421–425. doi: 10.1637/8574-010109-Reg.1. [DOI] [PubMed] [Google Scholar]
- 84.Fang Y., Reisen W.K. Previous infection with West Nile or St. Louis encephalitis viruses provides cross protection during reinfection in house finches. Am. J. Trop. Med. Hyg. 2006;75:480–485. [PubMed] [Google Scholar]
- 85.Gibbs S.E.J., Marlenee N.L., Romines J., Kavanaugh D., Corn J.L., Stallknecht D.E. Antibodies to West Nile virus in feral swine from Florida, Georgia, and Texas, USA. Vector-Borne Zoon. Diseases. 2006;6:261–265. doi: 10.1089/vbz.2006.6.261. [DOI] [PubMed] [Google Scholar]
- 86.United States Department of Agriculture - National Agricultural Statistics Service Map of Hog Inventory - Measured in Head (on Dec 1) 2023. https://app.usda-reports.penguinlabs.net/?crop=hogs&statistic=inventory_head&year=2023 (accessed July 15, 2024)
- 87.Nemeth N., Bosco-Lauth A., Oesterle P., Kohler D., Bowen R. North American birds as potential amplifying hosts of Japanese encephalitis virus. Am. J. Trop. Med. Hyg. 2012;87:760–767. doi: 10.4269/ajtmh.2012.12-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ladreyt H., Durand B., Dussart P., Chevalier V. How central is the domestic pig in the epidemiological cycle of Japanese encephalitis virus? A review of scientific evidence and implications for disease control. Viruses. 2019;11 doi: 10.3390/v11100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lord J.S., Gurley E.S., Pulliam J.R.C. Rethinking Japanese encephalitis virus transmission: a framework for implicating host and vector species. PLoS Neg. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.United States Department of Agriculture - National Agricultural Statistics Service Map of Broiler Production by State Million Head 2022. 2023. https://www.nass.usda.gov/Charts_and_Maps/Poultry/brlmap.php (accessed February 20, 2024)
- 91.Fagre A.C., Kading R.C. Can bats serve as reservoirs for arboviruses? Viruses. 2019;11 doi: 10.3390/v11030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lewis J.S., Farnsworth M.L., Burdett C.L., Theobald D.M., Gray M., Miller R.S. Biotic and abiotic factors predicting the global distribution and population density of an invasive large mammal. Sci. Rep. 2017;7 doi: 10.1038/srep44152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oliveira A.R.S., Cohnstaedt L.W., Noronha L.E., Mitzel D., McVey D.S., Cernicchiaro N. Perspectives regarding the risk of introduction of the Japanese encephalitis virus (JEV) in the United States. Front. Veterin. Sci. 2020;7 doi: 10.3389/fvets.2020.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.World Organisation for Animal Health . Terrestrial Animal Health Code. 2018. Chapter 2.1. Import risk analysis.https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=1&htmfile=chapitre_import_risk_analysis.htm [Google Scholar]
- 95.Rosen L., Lien J.-C., Shroyer D.A., Baker R.H., Lu L.-C. Experimental vertical transmission of Japanese encephalitis virus by Culex tritaeniorhynchus and other mosquitoes. Am. J. Trop. Med. Hyg. 1989;40:548–556. doi: 10.4269/ajtmh.1989.40.548. [DOI] [PubMed] [Google Scholar]
- 96.Van den Eynde C., Sohier C., Matthijs S., De Regge N. Japanese encephalitis virus interaction with mosquitoes: a review of vector competence, vector capacity and mosquito immunity. Pathogens. 2022;11 doi: 10.3390/pathogens11030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fluent Cargo. 2024. https://www.fluentcargo.com/ (accessed June 3, 2024)
- 98.Darsie R.F., Ward R.A. University Press of Florida Gainesville; FL: 2005. Others, Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. [Google Scholar]
- 99.Gorris M.E., Bartlow A.W., Temple S.D., Romero-Alvarez D., Shutt D.P., Fair J.M., Kaufeld K.A., Del Valle S.Y., Manore C.A. Updated distribution maps of predominant Culex mosquitoes across the Americas. Parasit. Vectors. 2021;14:547. doi: 10.1186/s13071-021-05051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Franckowiak Gregory A., Poché Richard M. Short-term home range and habitat selection by feral hogs in northern Texas. Am. Midl. Nat. 2018;179:28–37. doi: 10.1674/0003-0031-179.1.28. [DOI] [Google Scholar]
- 101.Peterson A.T., Vieglais D.A., Andreasen J.K. Migratory birds modeled as critical transport agents for West Nile virus in North America. Vector-Borne Zoon. Diseases. 2003;3:27–37. doi: 10.1089/153036603765627433. [DOI] [PubMed] [Google Scholar]
- 102.Swetnam D., Widen S.G., Wood T.G., Reyna M., Wilkerson L., Debboun M., Symonds D.A., Mead D.G., Beaty B.J., Guzman H., Tesh R.B., Barrett A.D.T. Terrestrial bird migration and West Nile virus circulation, United States. Emerg. Infect. Dis. 2018;24:2184–2194. doi: 10.3201/eid2412.180382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Redant V., Favoreel H.W., Dallmeier K., Van Campe W., De Regge N. Efficient control of Japanese encephalitis virus in the central nervous system of infected pigs occurs in the absence of a pronounced inflammatory immune response. J. Neuroinflammation. 2020;17:315. doi: 10.1186/s12974-020-01974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao S., Lou Y., Chiu A.P.Y., He D. Modelling the skip-and-resurgence of Japanese encephalitis epidemics in Hong Kong. J. Theor. Biol. 2018;454:1–10. doi: 10.1016/j.jtbi.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williams D.T., Mackenzie J.S., Bingham J. Flaviviruses. Diseases Swine. 2019:530–543. [Google Scholar]
- 106.Center for Disease Control and Prevention, Transmission of Japanese Encephalitis Virus. 2022. https://www.cdc.gov/japanese-encephalitis/php/transmission/?CDC_AAref_Val=https://www.cdc.gov/japaneseencephalitis/transmission/index.html
- 107.United States Department of Agriculture - Animal and Plant Inspection Service Importing Swine and Germplasm into the United States. 2024. https://www.aphis.usda.gov/live-animal-import/swine-germplasm (accessed January 4, 2023)
- 108.United States Department of Agriculture - Animal and Plant Inspection Service Importing Live Poultry into the United States. 2024. https://www.aphis.usda.gov/live-animal-import/live-poultry (accessed January 4, 2023)
- 109.Chen S., Jiang L., Zhong X., Zheng X., Ma S., Xiong Y., Zhou J., Li X., Ke X., Zhou W., Chen Q. Serological prevalence against Japanese encephalitis virus-Serocomplex Flaviviruses in commensal and Field rodents in South China. Vector-Borne Zoon. Diseases. 2016;16:777–780. doi: 10.1089/vbz.2015.1934. [DOI] [PubMed] [Google Scholar]
- 110.Miura K., Goto N., Suzuki H., Fujisaki Y. Strain difference of mouse in susceptibility to Japanese encephalitis virus infection. Exp. Anim. 1988;37:365–373. doi: 10.1538/expanim1978.37.4_365. [DOI] [PubMed] [Google Scholar]
- 111.Bosco-Lauth A., Mason G., Bowen R. Pathogenesis of Japanese encephalitis virus infection in a golden hamster model and evaluation of flavivirus cross-protective immunity. Am. J. Trop. Med. Hyg. 2011;84:727–732. doi: 10.4269/ajtmh.2011.11-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.United States Department of Agriculture - Animal and Plant Inspection Service How to Import Zoological Birds. 2024. https://www.aphis.usda.gov/live-animal-import/zoological-birds (accessed January 4, 2023)
- 113.United States Department of Agriculture - Animal and Plant Inspection Service Non-U.S. Origin Pet Birds Entering the U.S. From A Country Other Than Canada or Mexico. 2024. https://www.aphis.usda.gov/pet-travel/another-country-to-us-import/birds/non-us-origin (accessed January 4, 2023)
- 114.United States Food and Drug Administration IXIARO. 2019. https://www.fda.gov/vaccines-blood-biologics/vaccines/ixiaro (accessed January 4, 2023)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parameter inputs for FEVER and MINTRISK to qualitatively assess the incursion risk of JEV for seven US regions.
Median value and 95% uncertainty interval (UI) of the risk score for the annual rate of entry, the probability of transmission, the probability of establishment, and overall rate of introduction of Japanese encephalitis virus by introduction pathway into seven US regions at risk.
Data Availability Statement
The location of the publicly available datasets utilized in this study can be found cited in this article and supplemental material.