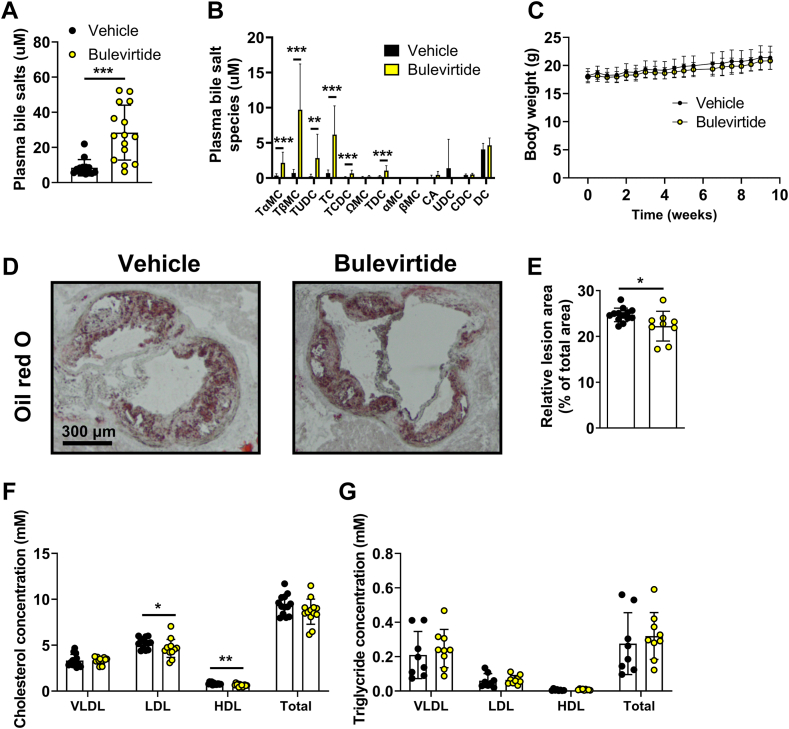
Fig. 2.
Bulevirtide treatment attenuates atherosclerosis development and lowers plasma cholesterol levels in Oatp1a1−/−Ldlr−/− mice. Female Oatp1a1−/−Ldlr−/− mice were injected subcutaneously with Na+ taurocholate co-transporting polypeptide (NTCP) inhibitor Bulevirtide (yellow circles or bars) or vehicle (black circles or bars) every day for 10 weeks. At study endpoint, (A) total plasma bile salt levels and (B) individual bile salt species were measured, including tauro-alpha-muricholic acid (TαMC), tauro-beta-muricholic acid (TβMC), tauroursodeoxycholic acid (TUDC), taurocholic acid (TC), taurochenodeoxycholic acid (TCDC), omega-muricholic acid (ΩMC), taurodeoxycholic acid (TDC), alpha-muricholic acid (αMC), beta-muricholic acid (βMC), cholic acid (CA), ursodeoxycholic acid (UDC), chenodeoxycholic acid (CDC), and deoxycholic acid (DC) (n = 14–15/group). C: Body weight was monitored throughout the study (n = 15–16/group). Cross-sections of the aortic root area were stained with Oil red O to visualize atherosclerotic lesions from which the area was determined. D: Representative images of each staining are displayed and (E) mean atherosclerotic lesion area was expressed relative to the total area of the aortic root (n = 9–14/group). Plasma (F) cholesterol (n = 13/group) and (G) triglyceride concentration (n = 8–9/group) was measured in very-low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and high-density lipoprotein (HDL). Data are presented as means ± SD. ∗Vehicle versus Bulevirtide. ∗ P < 0.05, according to Mann Whitney’s test (A), or student’s t test (B, E, F).