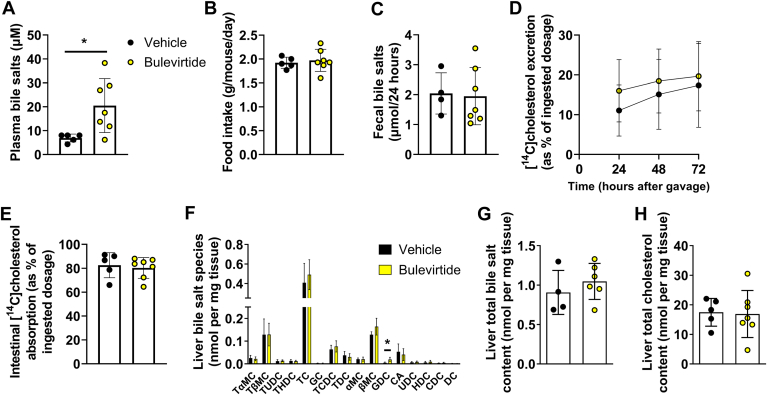
Fig. 4.
Bulevirtide treatment does not alter food intake, fecal bile salt levels, or intestinal cholesterol absorption in Oatp1a1−/−Ldlr−/− mice. Female Oatp1a1−/−Ldlr−/− mice were injected subcutaneously with Na+ taurocholate co-transporting polypeptide (NTCP) inhibitor Bulevirtide (yellow circles) or vehicle (black circles) every day for 3 days after administration of an oral bolus of [14C]cholesterol and [3H]sitostanol in olive oil. A: Plasma bile salts were measured after 3 days and (B) food intake was monitored throughout the 3 days (C) Bile salt levels were measured in feces collected during the last 24 h of treatment. 3H and 14C activity were determined in feces samples after 24, 48, and 72 h after administration of the tracers from which (D) fecal [14C]cholesterol excretion and (E) intestinal uptake were calculated. F: In the liver individual bile salt species were measured, including tauro-alpha-muricholic acid (TαMC), tauro-beta-muricholic acid (TβMC), tauroursodeoxycholic acid (TUDC), taurohyodeoxycholic acid (THDC), taurocholic acid (TC), glycocholic acid (GC), taurochenodeoxycholic acid (TCDC), taurodeoxycholic acid (TDC), alpha-muricholic acid (αMC), beta-muricholic acid (βMC), glycodeoxycholic acid (GDC), cholic acid (CA), ursodeoxycholic acid (UDC), hyodeoxycholic acid (HDC), chenodeoxycholic acid (CDC), and deoxycholic acid (DC). G: Total bile salt content was calculated by adding individual species. H: Hepatic total cholesterol content was measured. Data are presented as means ± SD (n = 4–7/group). ∗Vehicle versus Bulevirtide. ∗ P < 0.05, according to student’s t test (A) or two-way ANOVA (D).