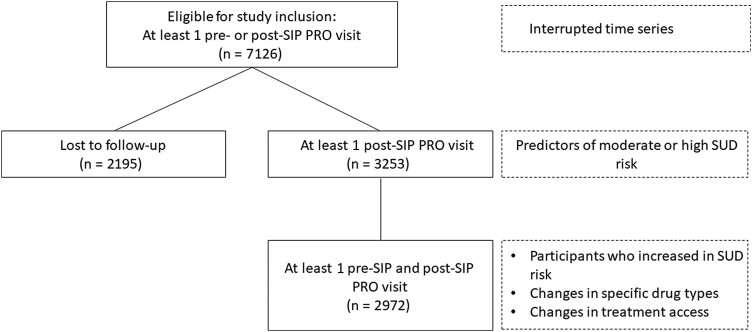
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram of participants included in each analysis. Subset of participants included in each analysis is presented in solid-line boxes, and specific analyses that used that subset of participants are presented in dashed-line boxes. Abbreviations: PRO, patient-reported outcomes and measures; SIP, shelter-in-place; SUD, substance use disorder.