Figure 2.
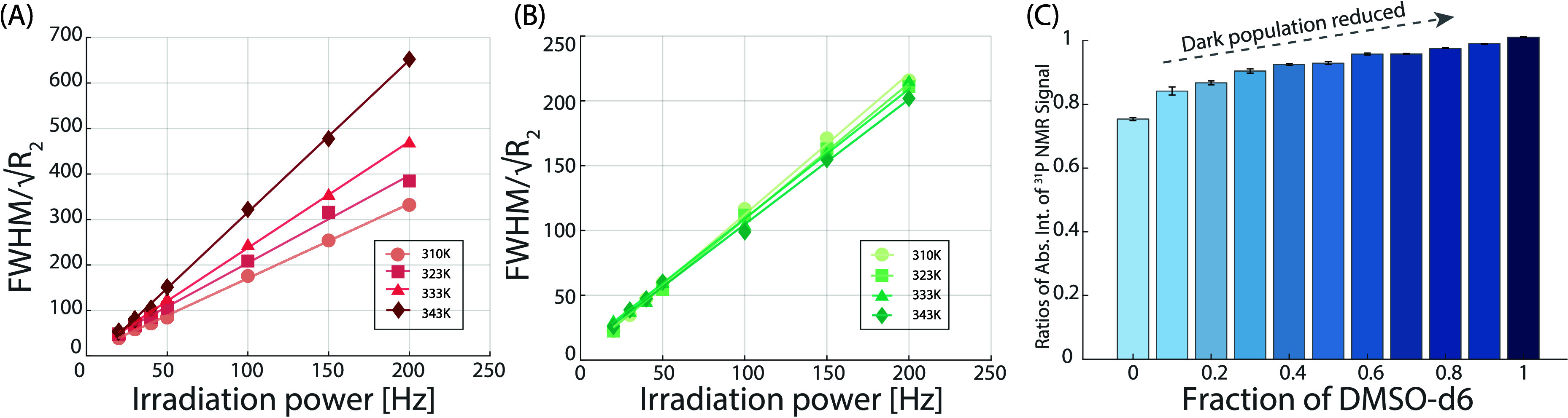
31P chemical
exchange analysis for 30 mM NADH in pure
deuterated solvents of DMSO-d6 and D2O. (A) Investigation of the irradiation power dependence on 31P CEST line widths, normalized by the square root of the
spin–spin relaxation rate (FWHM/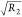 ), at temperatures of 310, 323, 333, and
343 K in pure D2O. The increasing slope observed at these
temperatures signifies an enhancement in the chemical exchange rate
with increasing temperature. (B) A parallel analysis conducted in
pure DMSO-d6. The uniform slopes across
the studied temperatures indicate a negligible chemical exchange in
this solvent. (C) Variation in the ratio of the absolute integral
of the 31P signal from 1D NMR spectra in 30 mM NADH solutions,
using binary mixed solvents of DMSO-d6 and D2O measured from 298 K to 343 K and adjusted for
the temperature dependence as discussed in the main text.. An increasing
ratio with a higher fraction of DMSO-d6 suggests that the presence of DMSO-d6 reduces the “dark” population of NADH in the solution.
), at temperatures of 310, 323, 333, and
343 K in pure D2O. The increasing slope observed at these
temperatures signifies an enhancement in the chemical exchange rate
with increasing temperature. (B) A parallel analysis conducted in
pure DMSO-d6. The uniform slopes across
the studied temperatures indicate a negligible chemical exchange in
this solvent. (C) Variation in the ratio of the absolute integral
of the 31P signal from 1D NMR spectra in 30 mM NADH solutions,
using binary mixed solvents of DMSO-d6 and D2O measured from 298 K to 343 K and adjusted for
the temperature dependence as discussed in the main text.. An increasing
ratio with a higher fraction of DMSO-d6 suggests that the presence of DMSO-d6 reduces the “dark” population of NADH in the solution.
