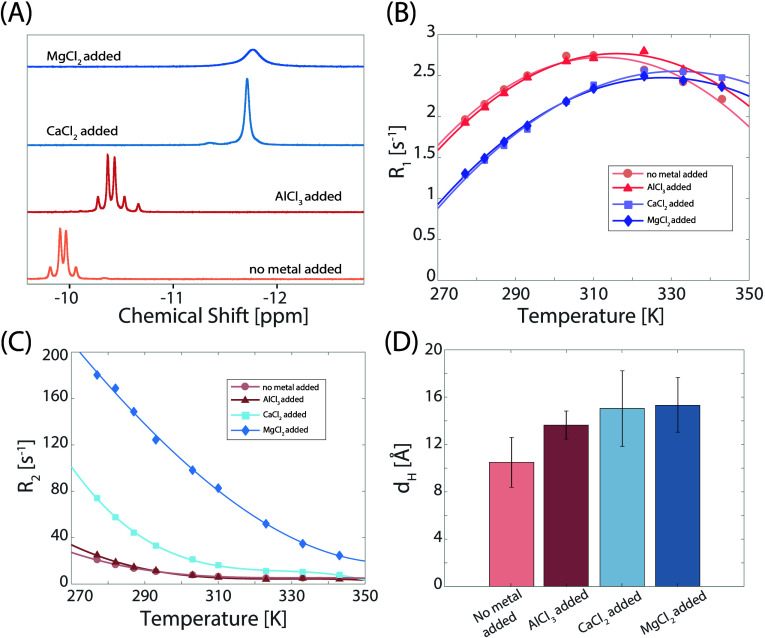
Figure 6.
31P NMR analysis of 30 mM NADH in 90% DMSO-d6 and 10% D2O, with and without 30 mM metal chloride salts. (A) 31P 1D NMR spectra variations: without metal chloride salts, the NADH peak appears as a doublet of doublets. Addition of 30 mM AlCl3 results in slight shielding (0.46 ppm) but retains the doublet of doublet structure. With 30 mM CaCl2, the peak transitions to a singlet with greater shielding (approximately 1.77 ppm), similar to the case with 30 mM MgCl2, though the MgCl2 added one has a broader line width. (B) Spin–lattice relaxation (R1) behavior: NADH shows a local maximum in R1 both with and without metal chloride salts added. The R1 profiles of NADH without metal and with AlCl3 track each other. The addition of CaCl2 or MgCl2 alters the R1 profile, delaying the appearance of the local maximum. (C) Spin–spin relaxation (R2) behavior: all R2 curves exhibit a monotonic decrease. The profiles in the absence of metal chloride salts and with AlCl3 are similar, while the addition of MgCl2 or CaCl2 significantly enlarges R2. (D) Hydrodynamic diameter (dH) extracted from the measured diffusion coefficients and dynamic viscosity in Figure 5A; all metal chloride salts increase NADH size, in the order of AlCl3 < CaCl2 < MgCl2.