Abstract
Cytauxzoon felis is a hemoprotozoan parasite of cats. While many infected cats die of acute illness, some enter a chronic carrier state. To date, no treatment has been documented to clear the chronic carrier state, leaving recovered cats to act as a potential indirect source of infection via a tick vector. Diminazene diaceturate is an anti-protozoal therapy that has been suggested for use in the treatment of acute cytauxzoonosis, but which failed to clear the carrier state at the dose used in acute illness. We hypothesized that a dose-intensified regimen of diminazene could reduce or eliminate parasitemia from five domestic cats naturally infected with C felis. Cats were administered 4 mg/kg of diminazene diaceturate intramuscularly for 5 consecutive days. Clearance of the organism was assessed via semi-quantitative polymerase chain reaction and light microscopy 1, 3, 6 and 10 weeks after starting treatment. Additionally, cats were monitored for adverse drug reactions by daily observation and examination. Complete blood count, biochemical profile and urinalysis were performed at 1, 3 and 10 weeks. Adverse events were common and included profuse salivation and nausea at the time of injection, monoparesis in the injected leg, proteinuria and potential hepatotoxicity. Severity of parasitemia was not reduced. Diminazene diaceturate cannot be recommended for elimination of the carrier state of C felis infection.
Introduction
Cytauxzoon felis is a hemoprotozoan parasite of felids that causes considerable morbidity and mortality. The acute phase of infection begins with schizogenous replication within the host mononuclear cells. Schizont distended macrophages can lead to widespread obstruction of the microvasculature with subsequent multisystem organ failure. Mononuclear cells eventually rupture, releasing merozoites that are then taken up by erythrocytes as piroplasms. 1
If a cat survives initial infection, piroplasms may persist within the erythrocytes for the life of the cat.2–4 These chronic carriers are capable of transmitting infection through the appropriate Amblyomma americanum tick vector. 5 Thus far, the only means of prevention relies on conscientious administration of ectoparasiticides, but even cats treated with parasiticides and those kept indoors can become infected. 6 Owners of chronically infected cats, often kept outdoors, may be reluctant to keep them as pets knowing that they might serve as a resevoir of infection.
Diminazene, an aromatic diamidine produced as either of two salts, has been used to treat a variety of protozoal diseases in domestic livestock worldwide. Although the mechanism of action is poorly understood, these compounds are thought to work via binding to the minor groove of parasite DNA. 7 In a case report of six cats with acute cytauxzoonosis treated with diminazene aceturate at 2 mg/kg intramuscularly, five cats survived. 8 Although the drug is not approved by the US Food and Drug Administration for use in any species in the USA, it can be obtained for a specific animal patient under Compassionate Use Protocols. While such importation is impractical for cats suffering acute cytauxzoonosis, it should be possible for the treatment of chronic carriers were it to be demonstrated to be efficacious.
Recently, we documented that at a dose regimen of diminazene similar to that used in the single published case series for the treatment of acute cytauxzoonosis was unable to reduce parasite burden of chronically C felis-infected cats, but adverse effects were minimal. 9 A more intense dose regimen of diminazene aceturate was recently demonstrated to eliminate Trypanosoma evansi from the majority of experimentally infected cats. 10 We hypothesized that an intensified dosing protocol of diminazene could reduce or eliminate parasitemia in naturally infected cats with chronic C felis infection, thus reducing their ability to act as a potential reservoir of infection.
Materials and methods
Animals
The study was approved by the University of Missouri Animal Use and Care Committee (protocol number 7174), and cats were group housed in an accredited facility and cared for according to the principles outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Five adult cats (two female spayed, three male castrated), all from the same region of northern Arkansas, were donated for the study of cytauxzoonosis by their owner after they were confirmed to be carriers of C felis. The middle-aged cats were all acquired as adults, had been owned for 2–5 years, and ranged in weight from 5.1 to 7.3 kg (median 5.7 kg ± 0.96 kg). All had been vaccinated for feline viral rhinotracheitis, calcivirus, and panleukopenia and rabies, and none had a known history of illness. At the time of donation, each cat underwent a complete physical examination, complete blood count (CBC), biochemical panel, C felis polymerase chain reaction (PCR), and feline leukemia and feline immunodeficiency virus antigen and antibody (respectively) testing. Cats were administered an anti-helminthic (fenbendazole 50 mg/kg PO or emodepside/ praziquantel topically 15 mg/60 mg per cat) and topical ectoparasiticide (fipronil) at donation. Before use in this dose-intense study, these cats had been previously treated with two injections of 3 mg/kg diminazene diaceturate given 1 week apart as part of a previously published study. 9 That treatment failed to reduce parasitemia as determined by PCR and light microscopic review of stained blood smears. 9 The last dose of diminazene had been administered 3.5 months prior to enrollment in this dose-intense study.
Treatment
A commercial preparation of diminazene diaceturate (70 mg/ml) and phenazone (375 mg/ml) in sterile water (Veriben RTU; Ceva Sante Animale) was obtained and stored as per manufacturer directions. Cats were premedicated with 0.03 mg/kg atropine 15 mins before injection of a 4 mg/kg intramuscular dose of diminazene diaceturate q24h h for a total of five doses. On the first day of treatment, the entire volume (0.29–0.42 ml) was administered in the biceps femoris, but after two cats developed monoparesis in the injected limb, injections were divided equally between the epaxial muscles and the quadriceps on days 2–5 of treatment. Owing to excessive vomiting and ptylism despite atropine premedication, 1 mg/kg of maropitant (Cerenia; Pfizer) was administered subcutaneously 30 mins prior to diminazene injection on days 3–5 of the study.
Clinical and laboratory evaluation
A baseline CBC with quantification of erythrocyte piroplasms, plasma biochemical panel, urinalysis and semi-quantitative PCR of blood for C felis were obtained just prior to study entry. Respiratory rate, respiratory effort and heart rate were recorded just before and every 15 mins for 2 h after injection. Cats were monitored for attitude, as well as injection site reactions, twice daily for 6 days, and then daily for the following 9 weeks. CBC with quantification of erythrocyte piroplasms, biochemical panel, urinalysis and PCR of blood for C felis were repeated at weeks 1, 3 and 10, while CBC with piroplasm quantification alone was performed at week 6. All cats had echocardiograms performed by a board-certified veterinary cardiologist after the study was completed.
CBC, biochemical panel and urinalysis were performed in the University of Missouri Veterinary Medical Diagnostic Laboratory in a routine fashion. Additionally, a trained technician reviewed Wright-Giemsa-stained blood smears. After thorough slide review, the number of piroplasm-infected erythrocytes per 1500 erythrocytes was counted.
PCR on ethylenediamine tetra-acetic acid-anticoagulated blood was performed using a previously published technique. 11 Briefly, each reaction consisted of 12.5 µl 2× PCR master mix (SsoFast EvaGreen Supermix; Bio-Rad), 7 μl water, 50 pmol of each oligonucleotide primer and 5 µl sample. Thermal cycling conditions were 98°C for 30 s followed by 45 cycles at 95°C for 5 s and 60°C for 5 s. Melting curve analysis was initiated at 75°C and data were captured at increasing increments of 0.5°C for 30 time points. For each PCR assay, positive (previously characterized C felis samples) and negative (no template) controls were used. Cycle threshold (Ct) determined from real-time PCR was used as an estimate of parasitemia. Typical precautions (eg, disposable gloves, clean-to-dirty workflow, and physical separation of pre- and post-PCR samples) were used to prevent amplicon carryover.
Statistical analysis
A one-way repeated measures analysis of variance was used to look for changes in both the number of infected erythrocytes observed microscopically and Ct PCR after treatment. Data met criteria for normalcy and equal variance. Calculations were performed by standard statistical software (SAS v9; SAS Institute). A P-value <0.05 was considered statistically significant.
Results
Adverse reactions and toxicity
Adverse reactions were common. Despite the use of atropine premedication, three cats demonstrated severe ptyalism soon after injection and four cats experienced repeated vomiting in the hours following initial injections. All the cats appeared nauseous and lethargic for several hours after injection, but the addition of maropitant pretreatment on days 3–5 resulted in dramatically less salivation and eliminated post-injection vomiting. Additionally, during the 10 weeks of observation a small amount of vomitus was found in the cats’ housing on an occasional basis. Three cats were painful on palpation near the sites of injection for 1–2 days after the first injection. All cats continued to eat and drink normally, and two of the three continued jumping onto play structures with apparent ease; therefore, analgesic medications were not administered. Two cats, including one cat that resented palpation of the injection site, developed mild-to-moderate monoparesis in the injected leg after the first injection. In one cat, paresis resolved within 24 h, but in the other cat the monoparesis persisted and improved gradually over months. The cat with long-lasting paresis did not seem to be uncomfortable on manipulation of the limb after the first day.
Hematologic values changed little during treatment. Neutropenia (segmented neutrophils 1.55 × 103/μl; reference interval 2.5–12.5 × 103/μl) was noted in one cat 10 weeks after treatment, but this same cat had demonstrated neutrophil counts either below or at the bottom of the reference interval since acquisition 10 months previously. Anemia was not identified in any cat at any time point (mean hematocrit at baseline 36.8% ± 4.5; mean hematocrit from all time points 37.4% ± 4.6; reference interval 24–45%). Platelet clumping prevented accurate enumeration of platelet counts on several samples, but estimated platelet numbers were always considered adequate.
Serum alanine aminotransferase (ALT) increased in four of five cats after treatment (Figure 1). Two cats were noted to have diminished appetite and to have lost body weight at weeks 5–6 after treatment. It was discovered that all cats had been transitioned to a new brand of commercial dry food abruptly during the second week of the study without our knowledge. At week six, serum ALT levels in the two cats with weight loss were 88 U/l and 238 U/l (reference interval 18–77), serum alkaline phosphatase levels were 75 U/l and 193 U/l (reference interval 5–55) and bilirubin levels were 0.2 and 2.0 mg/dl (reference interval 0–0.3), respectively. All five cats were transitioned back to the original diet; the cat with normal serum bilirubin gained weight rapidly, but the cat with hyperbilirubinemia failed to improve over the next few days and became completely anorexic. Biochemical abnormalities, abdominal ultrasound findings and hepatic cytology all supported a diagnosis of hepatic lipidosis in this cat. The cat improved rapidly after an esophagostomy tube was placed on day 47 to facilitate feeding on a high-calorie prescription diet (a/d; Hill’s Pet Nutrition); the feeding tube was removed after 10 days.
Figure 1.
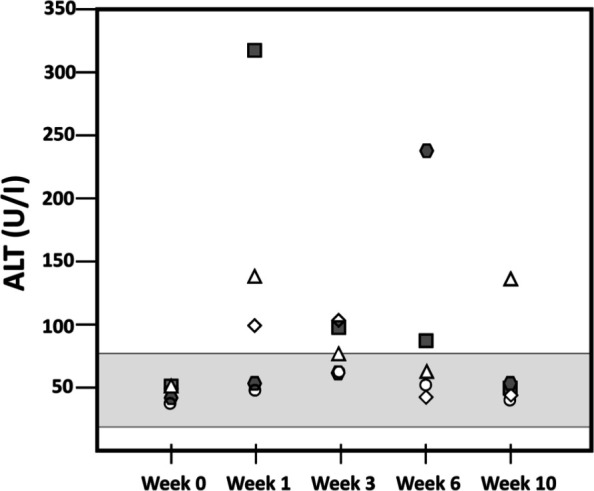
Plasma alanine aminotransferase (ALT) of each cat at 0, 1, 3, 6 and 10 weeks after injection of 4 mg/kg diminazene diaceturate intramuscularly. The area in the light-gray rectangle represents the reference interval for ALT. The cats represented by the colored square and hexagon are the two diagnosed with suspected mild or severe hepatic lipidosis, respectively
Every cat had well-concentrated urine at every time point (mean urine specific gravity 1.068 ± 0.009). Despite benign urine sediment, moderate proteinuria, as indicated by 2+ dipstick and sulfosalicylic acid of 50 mg/dl, was detected in two cats during the third week, but resolved thereafter. In a single cat, an unusual rhomboidal crystal was identified in the urine at week 10.
Echocardiography revealed one cat with each of the following findings: focal septal hypertrophy, tricuspid regurgitation, an enlarged left atrium and a structurally normal heart. Two cats had increased moderator bands and a left anterior fascicular block.
Parasitemia
Parasitized erythrocytes were evident microscopically from every cat just prior to study entry, but only at very low numbers (≤2 per 1500 erythrocytes). Parasites could not be identified after thorough slide review from two cats at the third week after treatment, but were again identified from these same cats at weeks 6 and 10. Parasites were observed on all other blood smear samples, always at low numbers. Compared with baseline, the average number of piroplasms noted per 1500 erythrocytes was not significantly different at 1, 3, 6 or 10 weeks, with a range of 0–2 infected erythrocytes per 1500 erythrocytes (P = 0.29).
All cats were PCR-positive for C felis at every time point. Cycle threshold values, which inversely correspond with parasite burden, did not statistically differ from baseline (Figure 2) (P = 0.05).
Figure 2.
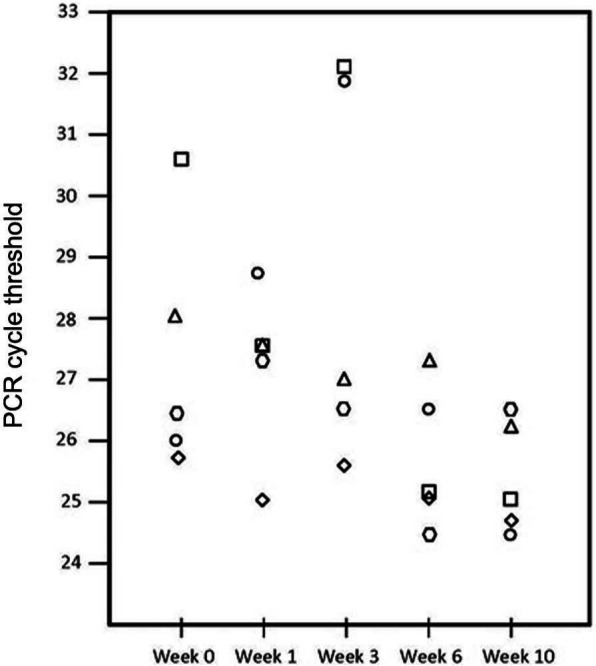
Mean cycle threshold (Ct) polymerase chain reaction (PCR) for all cats at 0, 1, 3, 6 and 10 weeks after injection of 4 mg/kg diminazene diaceturate intramuscularly. Lower Ct values roughly correspond with higher numbers of parasites. There was no statistical difference in parasite burden after treatment
Discussion
A high-dose, dose-intense protocol of diminazene administration was unable to reduce the parasite burden in cats that were chronic C felis carriers. In order to decrease the possibility that either microscopy or PCR would detect non-viable parasites in erythrocytes that had simply not yet been removed from circulation, cats were followed for 70 days, equivalent to the life-span of feline erythrocytes. 12 The degree of parasitemia did not vary over time, suggesting that relatively constantly low levels of parasitemia are present in cats with chronic C felis infection.
Compared with the few prior published studies of diminazene use in cats, we noted more adverse events.8–10,13 Although most of the previous reports used a lower dose administered less often, our study used an equivalent dose of diminazene base as used for the treatment of trypanosomiasis. 10 In that study, hepatic and renal parameters remained normal when tested every 14 days for 49 days after drug administration to seven cats. Although that study used diminazene aceturate (3.5 mg/kg, equivalent to 3.99 mg/kg diminazene diaceturate) rather than diminazene diaceturate (4 mg/kg, equivalent to 3.51 mg/kg diminazene aceturate) as a source of base drug, the dose of diminazene base was essentially the same. Few studies have compared aceturate and diaceturate preparations for efficacy or safety in any species. 14 The diaceturate product used in our study contained an antipyretic analgesic not found in the aceturate product, but each cat received only a total of 20 mg phenozone; this low-dose non-steroidal anti-inflammatory drug is unlikely to have caused any of the noted adverse effects. Only minor adverse events were identified in cats using the same diminazene diaceturate preparation used in the current study when administered at a lower dose.9,13 Alternatively, there may be differences inherent to the cats treated (eg, infection with Trypanosoma species versus Cytauxzoon species) that might explain the greater incidence of adverse events seen in our study compared with a previous study using an equivalently high dose.
Many cats in this study developed marked ptyalism and emesis. At a lower dosage, the same cats had hypersalivation alleviated by atropine, but no vomiting was observed. 9 At the higher dosage both hypersalivation and vomiting occurred despite atropine pretreatment, suggesting that these adverse events are due to more than just parasympathomemetic drug effects. The combination of atropine and maropitant pretreatment dramatically lessened salivation and eliminated vomiting. Because the neurokinin type 1 selective receptor antagonist maropitant inhibits vomiting due to multiple types of stimuli, this effect does not clarify the mechanism for vomiting in the cats of our study. 15 Both vomiting and hypersalivation have been reported after diminazene administration in other species.16–18
The observed monoparesis in two cats was thought to be precipitated by inadvertent injection of the drug into the sciatic nerve. Because it involved only the injected leg and occurred very soon after injection a peripheral neurotoxicity seemed far less likely, although nervous system toxicity has been reported in dogs treated with diminazene. 19 It is possible that the drug caused direct damage to the skeletal muscle. Diminazene has been reported to be toxic in vitro to cultured rat myocyte cells, skeletal muscle edema has been noted in dogs administered the drug and muscle necrosis has been identified at the site of intramuscular injection in cattle.20–22 No additional paresis was observed after dosing was altered to be given in two equivalent volumes in the quadriceps and epaxial musculature.
Hepatic enzyme activities were increased in most cats after diminazene administration. In two cats, hepatic lipidosis was either confirmed or suspected based on clinical findings, including resolution of weight loss and improvement in hepatic enzyme activities upon return to feeding. It was unfortunate that the cats’ diet was changed during the study period, making it difficult to know if anorexia, weight loss and hepatopathy were drug-related or due to the abrupt diet change. 23 Although weight loss was first noticed a few weeks after the diet change, group housing and feeding likely delayed recognition of the appetite reduction in the two affected cats. Review of other husbandry practices during this time period did not reveal any other variances from the acclimation period. In other species, xenobiotic toxicosis has been reported to cause histologic evidence of hepatic lipid accumulation, although, to our knowledge, this has not been reported in cats. 24 Although Da Silva et al 10 did not identify increased hepatic enzyme activities in cats treated with an equivalent dose of diminazene, the drug does result in increased hepatic enzyme activities in dogs and other species.17,25 Diminazene is sequestered in the liver and accumulates there for prolonged periods in dogs, rabbits and cattle.17,26,27 Our observation of increased serum ALT even in two cats with no change in appetite or weight loss suggests that the drug may at least result in hepatic enzyme activity increases even if it does not cause overt hepatic damage.
Two cats demonstrated a transient proteinuria while serum albumin remained normal. Other markers of renal injury, such as tubular casts, glucosuria, pyruria or loss of concentrating ability were not noted. The significance of the described rhomboid crystal is unclear. Abnormal urinary crystals have been reported with toxins such as ethylene glycol and the combination of melamine and cyanuric acid. 28 Additional doses would have to be given in a larger population to determine if crystalluria is, indeed, a side effect of diminazene diaceturate administration.
The only hematological abnormality was neutropenia in a single cat that had consistently demonstrated neutrophil counts at or below the lower reference interval during the 10 months since its acquisition and before receiving diminazene diaceturate. Prior to entry into this study, the cat had tested negative for feline leukemia virus by enzyme-linked immunosorbent assay (ELISA) and immunofluorescence antibody of bone marrow, negative for feline immunodeficiency virus antibody by ELISA and negative for Ehrlichia species by PCR. 9 A bone marrow examination revealed marked myeloid hypoplasia without an apparent cause. Despite periodic neutropenia, the cat has remained healthy. Diminazene has not previously been reported to cause hematologic abnormalities in any of several species.10,16,29,30 The neutropenia observed in this single cat is unlikely to be related to diminazene administration.
Because we identified cardiomyopathy in a single cat treated with two injections of 3.0 mg/kg diminazene diaceturate in our previously published study, all cats in the current study were examined by echocardiography. 9 Unfortunately, baseline cardiac investigation was not performed as cardiotoxicity has not been previously reported in association with diminazene use. Minor echocardiographic abnormalities were found in 4/5 cats. Given the heterogeneity of the abnormalities, it is likely that these simply represent a spectrum of spontaneous (naturally) acquired disease and are unlikely to represent a drug-induced cardiomyopathy.
This study included a small number of infected cats that had previously received two doses of diminazene. Strains of several haemoprotozoan parasites (eg, Trypanosoma species) in Africa have developed resistance to diminazene, but resistance has come about after 50 years of routine use of the drug in infected animals.31,32Although the mechanisms of protozoal resistance to diminazene are not entirely known, it seems extremely unlikely that the C felis in our cats would have developed drug resistance after the administration of only two doses. It is possible that our study was subject to type 2 error due to the small number of cats used, and that had more cats been included, some reduction of parasite burden may have been identified. However, as the most biologically important endpoint would be elimination of the organism, our findings would be largely unchanged.
Infection with C felis causes acute disease characterized by substantial morbidity and mortality. Although diminazene failed to clear infection of chronic carriers, it may still be effective for the treatment of acute disease. Greene et al 8 reported that 5/6 cats treated with diminazene aceturate for acute cytauxzoonosis survived the disease. The ability of an antiprotozoal to eliminate chronic parasitemia may not be necessary for it to result in improved survival from acute cytauxzoonosis. Indeed, the most effective treatment to date for acute infection — azithromycin and atovaquone — was ineffective at clearing the chronic parasitemia.6,33
Conclusions
Diminazene diaceturate failed to eliminate C felis from chronically infected carrier cats. At a dose of 4 mg/kg given intramuscularly for 5 consecutive days, cats experienced multiple adverse effects, including vomiting, salivation, injection site soreness, monoparesis, proteinuria and, perhaps, hepatotoxicity. Additional studies are warranted to investigate the use of diminazene diaceturate for the treatment of acute cytauxzoonosis, but treatment of chronic carrier cats with diminazene cannot be advocated.
Acknowledgments
We wish to thank Matt Haight, RVT and Dr John Dodam for their assistance with sample collection and statistical analysis, respectively.
Footnotes
Funding: Funding for this study was provided by a 501(c)(3) charitable foundation that wishes to remain anonymous.
The authors do not have any potential conflicts of interest to declare.
Accepted: 29 July 2013
This manuscript represents a portion of a thesis submitted by Dr Lewis to the University of Missouri Department of Veterinary Medicine and Surgery as partial fulfillment of the requirements for a MSc degree. Presented in abstract form at the 2012 ACVIM Forum, New Orleans, LA, USA June 2012
References
- 1. Cohn LA, Birkenheuer AJ. Cytauxzoonosis. In: Greene C. (ed). Infectious diseases of the dog and cat. St Louis, MO: Saunders Elsevier, 2011, pp 764–770. [Google Scholar]
- 2. Brown HM, Latimer KS, Erikson LE, et al. Detection of persistent Cytauxzoon felis infection by polymerase chain reaction in three asymptomatic domestic cats. J Vet Diag Invest 2008; 20: 485–488. [DOI] [PubMed] [Google Scholar]
- 3. Haber MD, Tucker MD, Marr HS, et al. The detection of Cytauxzoon felis in apparently healthy free-roaming cats in the USA. Vet Parasit 2007; 146: 316–320. [DOI] [PubMed] [Google Scholar]
- 4. Meinkoth J, Kocan AA, Whitworth L, et al. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997–1998). J Vet Intern Med 2000; 14: 521–525. [DOI] [PubMed] [Google Scholar]
- 5. Reichard MV, Edwards AC, Meinkoth JH, et al. Confirmation of Amblyomma americanum (Acari: Ixodidae) as a vector for Cytauxzoon felis (Piroplasmorida: Theileriidae) to domestic cats. J Med Entomol 2010; 47: 890–896. [DOI] [PubMed] [Google Scholar]
- 6. Cohn LA, Birkenheuer AJ, Brunker JD, et al. Efficacy of atovaquone and azithromycin or imidocarb dipropionate in cats with acute cytauxzoonosis. J Vet Intern Med 2011; 25: 55–60. [DOI] [PubMed] [Google Scholar]
- 7. Barrett MP, Gemmell CG, Sucling CJ. Minor groove binders as anti-infective agents. Pharmacol Ther 2013; 139: 12–23. [DOI] [PubMed] [Google Scholar]
- 8. Greene CE, Latimer K, Hopper E, et al. Administration of diminazene aceturate or imidocarb dipropionate for treatment of cytauxzoonosis in cats. J Am Vet Med Assoc 1999; 215: 497–500. [PubMed] [Google Scholar]
- 9. Lewis KM, Cohn LA, Marr HS, Birkenheuer AJ. Diminazene diaceturate for treatment of chronic Cytauxzoon felis parasitemia in naturally infected cats. J Vet Intern Med 2013; 26: 1490–1493. [DOI] [PubMed] [Google Scholar]
- 10. Da Silva AS, Zanette RA, Wolkmer P, et al. Diminazene aceturate in the control of Trypanosoma evansi infection in cats. Vet Parasitol 2009; 165: 47–50. [DOI] [PubMed] [Google Scholar]
- 11. Birkenheuer AJ, Le JA, Valenzisi AM, et al. Cytauxzoon felis infection in cats in the mid-Atlantic states: 34 cases (1998–2004). J Am Vet Med Assoc 2006; 228: 568–571. [DOI] [PubMed] [Google Scholar]
- 12. Kaneko JJ, Green RA, Mia AS. Erythrocyte survival in the cat as determined by glycine-2-C14. Proc Soc Exp Biol Med 1966; 123: 783–784. [DOI] [PubMed] [Google Scholar]
- 13. Lewis KM, Cohn LA, Birkenheuer AJ, Papich MG. Pharmacokinetics of diminazene diaceturate in healthy cats. J Vet Pharmacol Ther 2012; 35: 608–610. [DOI] [PubMed] [Google Scholar]
- 14. Rashid HB, Chaudhry M, Rashid H, et al. Comparative efficacy of diminazene diaceturate and diminazene aceturate for the treatment of babesiosis in horses. Trop Anim Health Prod 2008; 40: 463–467. [DOI] [PubMed] [Google Scholar]
- 15. Hickman MA, Cox SR, Mahabir S, et al. Safety, pharmacokinetics and use of the novel NK-1 receptor antagonist maropitant (Cerenia) for the prevention of emesis and motion sickness in cats. J Vet Pharmacol Ther 2008; 31: 220–229. [DOI] [PubMed] [Google Scholar]
- 16. Homeida AM, El Amin EA, Adam SE, Mahmoud MM. Toxicity of diminazene aceturate (Berenil) to camels. J Comp Pathol 1981; 91: 355–360. [DOI] [PubMed] [Google Scholar]
- 17. Miller DM, Swan GE, Lobetti RG, Jacobson LS. The pharmacokinetics of diminazene aceturate after intramuscular administration in healthy dogs. J S Afr Vet Assoc 2005; 76: 146–150. [DOI] [PubMed] [Google Scholar]
- 18. Kettner F. A study of the population pharmacokinetics of diminazene in dogs naturally infected with Babesia canis. MMedVet (Med) Thesis. University of Pretoria, South Africa, 2007. [Google Scholar]
- 19. Naude TW, Basson PA, Pienaar JG. Experimental diamidine poisoning due to commonly used babecides. Onderstepoort J Vet Res 1970; 37: 173–184. [PubMed] [Google Scholar]
- 20. Fairclough R. Observations on the use of Berenil against against trypanosomiasis of cattle in Kenya. Vet Rec 1963; 75: 1107–1112. [Google Scholar]
- 21. Gillingwater K, Kumar A, Ismail MA, et al. In vitro activity and preliminary toxicity of various diamidine compounds against Trypanosoma evansi. Vet Parasitol 2010; 169: 264–272. [DOI] [PubMed] [Google Scholar]
- 22. Losos GJ, Crockett E. Toxicity of beril in the dog. Vet Rec 1969; 85: 196. [DOI] [PubMed] [Google Scholar]
- 23. Armstrong PJ, Blanchard G. Hepatic lipidosis in cats. Vet Clin North Am Small Anim Pract 2009; 39: 599–616. [DOI] [PubMed] [Google Scholar]
- 24. Amacher DE. Strategies for the early detection of drug-induced hepatic steatosis in preclinical drug safety evaluation studies. Toxicology 2011; 279: 10–18. [DOI] [PubMed] [Google Scholar]
- 25. Akpa PO, Ezeokonkwo RC, Eze CA, Anene BM. Comparative efficacy assessment of pentamidine isethionate and diminazene aceturate in the chemotherapy of Trypanosoma brucei brucei infection in dogs. Vet Parasitol 2008; 151: 139–149. [DOI] [PubMed] [Google Scholar]
- 26. Kellner HM, Eckert HG, Volz MH. Studies in cattle on the disposition of the anti-trypanosomal drug diminazene diaceturate (Berenil). Trop Med Parasitol 1985; 36: 199–204. [PubMed] [Google Scholar]
- 27. Gilbert RJ. Studies in rabbits on the disposition and trypanocidal activity of the anti-trypanosomal drug, diminazene aceturate (Berenil). Br J Pharmacol 1983; 80: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thompson ME, Lewin-Smith MR, Kalasinsky VF, et al. Characterization of melamine-containing and calcium oxalate crystals in three dogs with suspected pet food-induced nephrotoxicosis. Vet Pathol 2008; 45: 417–426. [DOI] [PubMed] [Google Scholar]
- 29. Peregrine AS, Mamman M. Pharmacology of diminazene: a review. Acta Trop 1993; 54: 185–203. [DOI] [PubMed] [Google Scholar]
- 30. Tuntasuvan D, Jarabrum W, Viseshakul N, et al. Chemotherapy of surra in horses and mules with diminazene aceturate. Vet Parasitol 2003; 110: 227–233. [DOI] [PubMed] [Google Scholar]
- 31. Vitouley HS, Sidibe I, Bengaly Z, et al. Is trypanocidal drug resistance a threat for livestock health and production in endemic areas? Food for thoughts from Sahelian goats infected by Trypanosoma vivax in Bobo Dioulasso (Burkina Faso). Vet Parasitol 2012; 190: 349–354. [DOI] [PubMed] [Google Scholar]
- 32. Wickramasekara Rajapakshage BK, Yamasaki M, Hwang SJ, et al. Involvement of mitochondrial genes of Babesia gibsoni in resistance to diminazene aceturate. J Vet Med Sci 2012; 74: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 33. Cohn LA, Birkenheuer AJ, Ratcliff E. Comparison of two drug protocols for clearance of Cytauxzoon felis infections. J Vet Intern Med 2008; 22: 704. [Google Scholar]


