Abstract
The etiopathogenesis of feline inflammatory liver disease (ILD) is unclear. Therefore, we sought to determine the presence and distribution of bacteria within the livers of cats with ILD using eubacterial fluorescence in situ hybridization (FISH). Histopathology from 39 cats with ILD and 19 with histologically normal livers (C) were classified using World Small Animal Veterinary Association guidelines. Hepatic sections were examined by 16 and 23S ribosomal RNA FISH. Antibodies against cytokeratins and factor VIIIa were used to distinguish bile ducts and vascular structures. Histopathologic findings included non-specific reactive hepatitis (12), neutrophilic cholangitis (NC; 12), lymphocytic cholangitis (seven), cholestasis/obstruction (three), probable lymphoma (three) and acute hepatitis (two). Bacteria were observed in 21/39 ILD and 3/19 C (P = 0.0054). In 8/39 ILD and 2/19 C bacteria were restricted to the outer liver capsule (P = 0.29) and may represent contaminants. The prevalence of intrahepatic bacteria was higher (P = 0.008) in ILD (13/31) than C (1/17). Bacteria in ILD were more frequently (P <0.0001) localized to portal vessels, venous sinusoids and parenchyma (12/13) than bile duct (1/13). Bacterial colonization was highest in Escherichia coli-positive NC cats. Concurrent non-hepatic disease, predominantly pancreatic and intestinal (8/10 cats biopsied), was present in all 13 cats with intrahepatic bacteria. Bacterial culture was positive (predominantly E coli and Enterococcus species) in 11/23 (48%) samples, and concurred with FISH in 15/23 cases. The presence of intrahepatic bacteria in 13/31 (41%) cats with ILD suggests a role in etiopathogenesis. The distribution of bacteria within the liver supports the possibility of colonization via either enteric translocation or hematogenous seeding.
Introduction
Feline inflammatory liver disease (ILD) encompasses a group of acquired inflammatory disorders that are predominantly centered on the hepatobiliary tree (cholangitis) and less commonly associated with hepatic parenchymal inflammation (hepatitis). 1 Feline ILD has also been categorized as being either suppurative or non-suppurative to reflect the relative proportions of neutrophils to lymphocytes and plasma cells, and by the degree of bile duct hyperplasia and fibrosis. 2 More recently, a histological classification for liver disease has been proposed by the World Small Animal Veterinary Association (WSAVA) Liver Standardization Group. 3 They believe the most common inflammatory hepatic disorders of the cat involve the biliary system (cholangitis) and that inflammatory hepatic parenchymal disorders (hepatitis) are less common. The authors 3 describe four major subcategories of cholangitis: neutrophilic (acute or chronic) cholangitis (NC), lymphocytic cholangitis (LC), destructive cholangitis and cholangitis associated with liver fluke infestation. NC and LC are the most common forms of ILD in cats with destructive cholangitis and fluke infestation being rare. Non-specific reactive hepatitis (RH) is the response to a variety of extrahepatic disease processes with variable inflammatory and degenerative changes without hepatic necrosis, and is the most common parenchymal inflammatory condition observed in cats. Acute hepatitis (AH) represents the most common primary hepatic parenchymal inflammatory disorder. The causes of almost all feline ILD have not been determined, but it is suspected that infectious agents or immune mechanisms may underlie the inflammatory response in some of these cases.3–5
There are numerous reports of bacteria identified in various types of feline ILD, with enteric species such as Escherichia coli, Enterococcus species, Clostridium species and Salmonella species being most often cultured from the bile or hepatic tissue.6,7 However, the role bacteria play in feline ILD, or the specific types of ILD associated with bacteria, have not been well described. Helicobacter species are associated with cholangitis in humans and other species, and polymerase chain reaction (PCR) amplification has detected Helicobacter DNA in bile of 4/15 cats with LC and 5/51 with non-LC. 8 However, the detection of Helicobacter DNA in livers of only 2/32 cats with cholangitis/cholangiohepatitis and bile of 7/12 clinically healthy cats questions the significance of Helicobacter species in feline cholangitis. 4 A recent study using fluorescence in situ hybridization (FISH), which enables visualization of intact bacteria within tissues, found evidence of intrahepatic bacteria in only 2/36 cats with LC and 0/18 with lymphoma. 5 No studies to date have determined the presence or spatial distribution of bacteria in the liver of cats with NC, neutrophlic hepatitis, reactive hepatopathy or cholestasis. Further, concurrent intestinal and/or pancreatic disease commonly accompany ILD, and it is unknown if these diseases are at all linked with the presence of bacteria in feline ILD. 9
It is with this background in mind that we sought to determine the presence, type and location of intact bacteria within the livers of cats with various forms of ILD using FISH with oligonucleotide probes directed against bacterial 16 and 23S ribosomal RNA (rRNA). We also investigated whether there is a link between concurrent disease, such as intestinal or pancreatic disorders, and the presence of hepatic bacteria.
Materials and methods
Patient samples
The histopathology database from the Diagnostic Laboratory at Colorado State University was reviewed between 1999 to 2008 to identify liver biopsies that had a diagnosis consistent with ILD. Archived hematoxylin and eosin (HE)-stained histopathology slides were reviewed, and cases with histologic features that were inconsistent with a diagnosis of ILD were excluded. Cases identified as having a significant primary non-gastrointestinal (GI) disease were not included in this evaluation. Cases reported to have normal liver histology were also retrieved and reviewed to serve as normal, negative controls (C). Liver biopsies were either obtained as surgical wedge samples or as laparoscopic biopsies with biopsy cup forceps. Necropsy samples with histological evidence of autolysis were excluded from this study. Thirty-nine cases of ILD and 19 cases with normal histology (C) were identified. The archived paraffin-embedded tissue blocks from these cases were acquired for further analysis. The medical records and diagnostic reports of these cases were also reviewed for the results of liver or bile cultures, and for histological evidence of concurrent pancreatic, GI or other concurrent organ system diseases.
Histopathological scoring
Archival formalin-fixed paraffin-embedded tissue blocks were sectioned at 4 μm and stained with hematoxylin and eosin (HE) for initial histological scoring. Histopathology was classified according to WSAVA guidelines by a board-certified pathologist (JC) with sections allocated to one of the following groups: (1) NC characterized by neutrophils within the bile duct lumen or between biliary epithelial cells representing an acute form, or as a chronic form having mixed inflammatory infiltrates, including neutrophils, lymphocytes and plasma cells in the portal area, as well as possible fibrosis; (2) LC characterized by small lymphocytes surrounding bile ducts and usually with an associated biliary proliferation; (3) AH characterized by aggregates of parenchymal neutrophils associated with areas of hepatocellular apoptosis or necrosis; (4) non-specific RH characterized most often by light-to-moderate portal tract infiltrates of any combination of lymphocytes, macrophages, plasma cells, neutrophils or eosinophils that were distributed diffusely within the portal tract connective tissue or scattered within the parenchyma, but without hepatic necrosis; (5) cholestasis/obstruction (CO) characterized by the presence of plugged bile canaliculi, portal tract edema and light infiltrates of neutrophils or circumferential spindle cells surrounding bile ducts, portal tract fibrosis and mononuclear cell infiltrates in chronic cases; (6) probable lymphoma (prLSA) characterized by prominent lymphocyte infiltrates expanding the portal tract space, but without evidence of biliary hyperplasia.3,10 In all cases, the number of inflammatory cells (neutrophils, lymphocytes, plasma cells, macrophages), and the extent of peribiliary fibrosis and biliary hyperplasia were each scored independently. For cellular infiltrate grading an average of all portal tracts was determined subjectively for each biopsy. Normal (0) was characterized by fewer than five cells of each type evident; mild (1) was characterized by 5–25 cells within a portal tract; moderate (2) by 25–50 cells; and severe (3) by 50 or more cells per portal tract or having diffuse cellular infiltrates that extended beyond the limiting plate and into the parenchyma or bridging portal tracts (4). Fibrosis was scored as either normal (0), with no apparent increase in concentric periductular cells; mild (1) with 1–2 layers of surrounding cells; moderate (2) with 2–10 layers of surrounding cells; severe (3) with more than 10 layers of surrounding cells or diffuse expanding fibrosis extending from portal to portal area or into the adjacent parenchyma (4). Biliary hyperplasia was scored as either normal (0) with 1–3 interlobular bile ducts or smaller caliber ducts per portal tract; mild (1) with 4–6 duct profiles; moderate (2) with 6–10 duct profiles; or severe (3) with more than 10 duct profiles.
FISH and immunocytochemistry
Formalin-fixed paraffin-embedded histological sections (4 μm) were mounted on Probe-On Plus slides (Fisher Scientific) and evaluated by FISH as previously described. 11 Briefly, paraffin-embedded biopsy specimens were de-paraffinized by passage through xylene (3 × 10 mins), 100% alcohol (2 × 5 mins), 95% ethanol (5 mins) and, finally, 70% ethanol (5 mins). The slides were air-dried. FISH probes 5’-labeled with either Cy3 or 6-FAM (Integrated DNA Technologies) were reconstituted with sterile water and diluted to a working concentration of 5 ng/µl with a hybridization buffer appropriate to the probe. For initial evaluation EUB338 Cy-3 was combined with the irrelevant probe non-EUB-338-FAM (ACTCCTACGGGAGGCAGC) to control for non-specific hybridization. For subsequent analyses a specific bacterial probe labeled with Cy-3 and the universal bacterial probe EUB338 labeled with 6-FAM were applied simultaneously. Specific probes, directed against Clostridium species, Bacteroides/Prevotella species, Enterobacteriaceae, E coli, Helicobacter species and Streptococcus species were selected on the basis of historical association with feline ILD, their isolation in cultured liver biopsies and bacterial morphology on eubacterial FISH. Sections were allowed to hybridize with 30 µl of DNA probe mix in a hybridization chamber overnight (12–14 h). Washing was performed with the appropriate wash buffer (hybridization buffer without sodium dodecyl sulfate), and the samples were then rinsed in sterile water, allowed to air-dry, and mounted with ProLong Antifade Gold (Molecular Probes).
Probe specificity was controlled by evaluating slides prepared from cultured E coli DH5a, Salmonella typhimurium (ATCC14028), Proteus vulgaris, Enteroccocus fecium, Streptococcus equi, Streptococcus bovis, Clostridium perfringens, Clostridium difficile and Helicobacter pylori. Sections of gastric mucosa from cats or dogs with Helicobacter species infections were used as additional controls for Helicobacter species FISH. 12 Probe specificity was additionally evaluated by including positive and negative control slides in each assay. Sections were examined on an Olympus BX51 epifluorescence microscope and images captured with an Olympus DP-7camera (Olympus America).
The number, morphology and location of bacteria (capsular or within the liver), and their spatial distribution within the liver (sinusoids, bile duct, intravascular) was determined by visual examination of the entire biopsy sample. Precise localization of bacteria was further aided by co-staining with antibodies against cytokeratins and factor VIIIa to enable unequivocal distinction between bile ducts and vascular structures, respectively.
Briefly, tissue sections were de-paraffinized, rehydrated, washed in tris-buffered saline (TBS), and incubated with pre-diluted pepsin (Zymed/Invitrogen) for 50 mins at 37°C. The slides were then processed for EUB338-Cy3 in situ hybridization as described above. After washes with TBS, TBS 0.05% TritonX and TBS — each for 5 mins — the sections were blocked with 10% goat/10% horse serum/2× casein, for 20 mins at room temperature and blotted. Sections were then incubated with rabbit anti-Von Willebrand factor (vWF) antibody [polyclonal rabbit anti-human vWF (A0082, DAKO)] diluted 1:30 in a 1:20 dilution of mouse anti-keratin AE1/AE3 antibody (monoclonal mouse anti-human cytokeratin AE1/AE3, M 3515; DAKO) in TBS 1× casein, for 2 h at 37°C and washed three times in TBS. To detect vWF, sections were incubated with fluorescein isothiocyanate goat anti-rabbit IgG heavy and light chain (H&L) secondary antibody at 1:50 (Vector Laboratories) protected from light for 20 mins at room temperature and blotted/washed with TBS. 7-amino-4-methylcoumarin-3-acetic acid (AMCA)-conjugated horse anti-mouse IgG (H&L) (Vector Laboratories) was applied at 1:80 for 20 mins, at room temperature in the dark, to detect cytokeratin. Slides were washed and mounted with Vectashield 4’,6-diamidino-2-phenylindole (Vector Laboratories). Normal rabbit IgG (Vector Laboratories) and normal mouse IgG (1, 2a, 2b; Vector Laboratories) were substituted for primary antibodies at equivalent μg/ml (final dilution) to serve as negative controls.
Statistical analysis
Differences in age were compared using the unpaired Student’s t-test. Differences in the proportions of FISH-positive liver biopsies in ILD and control, different sample types (biopsy, necropsy) and histological categories of liver disease (CO, LC, LSA, NC, NH, RH, Control) were compared using Fisher’s exact test. Differences in the number of neutrophils, plasma cells, lymphocytes, macrophages and degree of periductular fibrosis between sections that were positive and negative for intrahepatic FISH-positive bacteria were evaluated using the Mann–Whitney U-test. A P-value of <0.05 was considered significant.
Results
Case samples
Thirty-nine cases with ILD (mean age 10.5 ± 3.9 SD years, range 2–17 years; 19 neutered males, two intact males and 18 neutered females) and 19 histologically normal feline control cases (mean age 5.3 ±3.8 SD years, range 0.25–13 years; 11 neutered males, one intact male, three intact females and four neutered females) were identified. Controls cats were younger than ILD cats (P <0.0001). There was no significant difference in gender distribution. Cats with ILD were domestic shorthair (DSH) (23), domestic longhair (DLH) (eight), Himalayan (two), Siamese (two), Manx (one), Maine Coon (one), Persian (one) and exotic (one). Control cats were DSH (14), DLH (two), Bengal (one), Himalayan (one) and Sphinx (one). The liver samples were collected either surgically or laparoscopically (27 ILD, 15 C) or during necropsy (12 ILD, four C). Twenty-six cases of ILD also had other tissues collected for histological examination, including small intestine (24), stomach (seven), pancreas (23), gallbladder (three) and mesenteric lymph nodes (21). Concurrent diseases identified on the histopathology reports of cats with ILD included pancreatitis (15: 12 chronic pancreatitis; two acute pancreatitis; one pancreatic carcinoma with chronic pancreatitis), inflammatory bowel disease (IBD; 11: eight lymphocytic plasmacytic enteritis; one severe eosinophilic enteritis; one severe suppurative colitis; one pyogranulomatous enteritis) and probable intestinal lymphoma (five). The eight cases of lymphocytic plasmacytic enteritis were histologically classified as either mild (three), moderate (three) or severe (two). Seven cases had both pancreatitis and IBD, and six cases had bile duct obstructions confirmed at surgery (three bile duct inflammation/fibrosis, two gastrointestinal neoplasia and one pancreatic neoplasia). Three control cases had concurrent IBD, all classified as mild lymphocytic plasmacytic enteritis. Twenty-three of the ILD cases had aerobic and anaerobic cultures of liver tissue, and one case had a concurrent bile culture. Eleven/23 (48%) ILD cases cultured were positive, and one case had a negative liver culture, but positive bile culture. The organisms identified were E coli (from from liver, one from bile), Enterococcus faecalis (three) Staphylococcus species (three), S bovis (one) and Actinomyces species (one), with the latter two considered likely contaminants. One of the cases positive for E faecalis also had positive cultures for coagulase negative Staphylococcus species, Staphylococcus aureus and C perfringens. No liver cultures were obtained in the control cats.
Histopathology
Histopathology was classified as non-specific RH (12), NC (12), LC (seven), CO (three), prLSA (three), or AH (two) or normal control (N; 19) (Table 1). Histological evidence of concurrent CO was also identified in NC (four), RH (one), and LC (one) classifications. Livers classified as CO had fewer inflammatory changes to place them into other categories, but had characteristic changes of biliary cholestasis or obstruction. The prLSA cases were thought to be most likely lymphoma, rather than severe LC as further confirming tests were not performed.
Table 1.
Relationship of histological subtype to the presence and spatial distribution of bacteria within the liver
| Histological classification (n) | FISH-positive | Bacterial location | |||
|---|---|---|---|---|---|
| Capsule | Parenchyma | Vascular | Bile duct | ||
| Reactive hepatitis (12) | 8 | 3 (2 Eu, 1 St) | 2 (Eu) | 3 (2 Eu, 1 Ec) | |
| Neutrophilic cholangitis (12) | 6* | 2 (2 Eu) | 2 (Ec) | 3 (Ec) | 1 (Ec) |
| Lymphocytic cholangitis (7) | 4 | 3 (Eu, En, Ec) | 1 (Ec) | ||
| Cholestasis/obstruction (3) | 1 | 1 (Ec) | |||
| Probable lymphoma (3) | 1 | 1 (Ec) | |||
| Acute hepatitis (2) | 2 | 1 (Eu) | 1 (Eu) | ||
| Normal (19) | 3 | 2 (Eu) | 1 (Eu) | ||
Eu = hybridized with EUB-338; St = hybridized with probe recognizing Streptococcus species;
Ec = hybridized with probe recognizing Escherichia coli ; En = hybridized with probe recognizing Enterococcus species
Two cats were co-colonized in vascular and parenchyma
FISH
Bacteria (EUB338) were observed more frequently (P = 0.0054) in ILD (21/39) than normal livers (C, 3/19) (Table 1). Bacteria in eight ILD (three RH, three LC, two NC) and two C were restricted to the outer surface of the liver capsule and thought to represent contaminants (Figure 1a). The prevalence of capsular bacteria was similar (P = 0.29) in ILD (8/39) and C (2/19). In contrast, the prevalence of intrahepatic bacteria was significantly higher in ILD than C when samples with capsular bacteria were considered contaminants, ie, intrahepatic bacteria in 13/39 ILD and 1/19 C (P = 0.017), or treated as ‘equivocal’ and excluded from the analysis, ie intrahepatic bacteria in 13/31 ILD and 1/17 C (P = 0.008).
Figure 1.
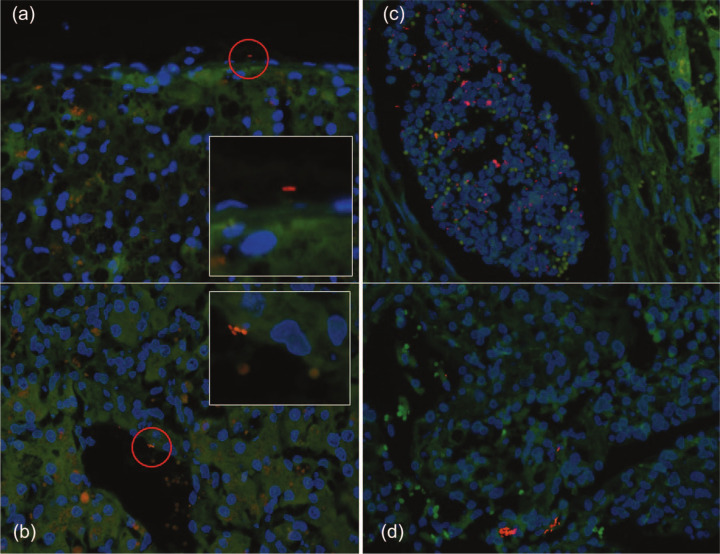
Regional distribution of bacteria in hepatic biopsies. (a) A bacterium (EUB338-Cy3, red) is visualized on the capsule of the liver (magnified in insert). (b) Bacteria (Escherichia coli-Cy3,red) within a hepatic sinusoid (magnified in insert). (c) Multiple bacteria (EUB338-Cy3, red) within a hepatic micro-abscess. (d) Multiple bacteria (EUB338-Cy3, red) within the hepatic parenchyma
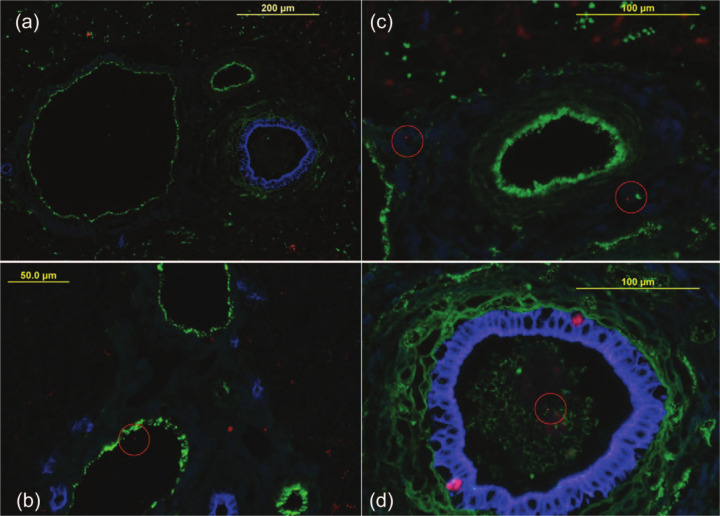
In cats with ILD intrahepatic bacteria were visualized within or around portal vessels, or venous sinusoids of nine (three NC, three RH, one AH, one prLSA, 1 CO; Figure 1b, Figure 2 b,c), the bile duct of one (NC; Figure 2d) and within the hepatic parenchyma near the capsule in cats (two NC one LC, one AH, two RH) cats (Figure 1d). Bacteria in ILD were more frequently (P <0.0001) localized to portal vessels or venous sinusoids or the parenchyma (12/13) than the bile duct (1/13). Bacterial colonization was highest in two cats with NC, one of which had hepatic micro-abscesses (Figure 1c), and the other had RH and a portal vascular anomaly. In the control cat with intrahepatic bacteria, bacteria were visualized around portal vessels and a venous sinusoid. This cat was biopsied surgically as part of the diagnostic investigation for unexplained lethargy, and the primary disease was undetermined. There was no significant difference in bacterial colonization between samples obtained from control cats at necropsy or surgery (P = 0.53).
Figure 2.
Localization of intrahepatic bacteria. A combination of eubacterial fluorescence in situ hybridization (EUB338-Cy3, red) and immunofluorescent staining of vascular endothelium (anti-Von Willebrand factor-fluorescien isothiocyanate, green) and bile duct epithelium (anti-human cytokeratin AE1/AE3-AMCA, blue) was used to enable precise localization of bacteria within the liver. (a) Vascular and biliary structures are clearly distinguished by green and blue staining of endothelium and epithelium, respectively. (b) A bacterium is visualized within a vascular/lymphatic lumen. (c) Two bacteria within the hepatic parenchyma surrounding portal triad. (d) A bacterium is visible within a bile duct lumen
Relationship of intrahepatic bacteria to histopathological phenotype
Intrahepatic bacteria were visualized in CO (1/3), LC (1/7), prLSA (1/3), NC (3/12), AH (2/2) and RH (5/12) (Table 1). There was no statistical association of FISH-positive bacteria with the individual histopathological category or the predominance of lymphocytes (prLSA+LC,2/10) versus neutrophils (AH + NC, 5/14).
Relationship of quantitative pathology to intrahepatic bacteria
The median numbers of neutrophils macrophages, plasma cells, lymphocyte and degree of periductular fibrosis were not significantly different in FISH-positive (intrahepatic bacteria) versus FISH-negative samples (Table 2). The number of plasma cells correlated with the degree of fibrosis (P = 0.031; rho 0.41).
Table 2.
The relationship of intrahepatic bacteria to quantitative histopathology
| FISH | Neutrophils | Plasma cells | Lymphocytes | Macrophages | Periductular fibrosis |
|---|---|---|---|---|---|
| FISH negative | 0.5 (0–3) | 0.5 (0–2) | 1 (1–4) | 0 (0–2) | 1 (0–4) |
| FISH positive | 0.5 (0–4) | 0 (0–3) | 1 (1–4) | 1 (0–2) | 0 (0–4) |
FISH = fluorescence in situ hybridization
Median and range given in parentheses
Correlation of FISH with bacterial culture
Twenty-three cases had hepatic cultures performed. There was no significant correlation between FISH and bacterial culture (Spearman’s rho 0.302, P >0.05). FISH and culture results concurred in 15/23 cases, with both FISH and culture being positive in six and both FISH and culture being negative in nine cases. Bacterial culture was positive in 7/21 FISH-positive samples (five E coli, three Enterococcus, two Staphylococcus, one Actinomyces, one Streptococcus). FISH or culture revealed E coli in 3/3 NC with invasive bacteria. In eight cases FISH did not correlate with culture: 3/6 FISH-positive samples had scant numbers of capsular bacteria and were culture-negative (Figure 1a). Five culture positive samples, including two for Staphylococcus species, were FISH-negative.
Relationship of bile duct obstruction to intrahepatic bacteria
Nine cases were grouped as bile duct obstructions based on either histological (three) or surgical (six) evidence of an obstruction. Histological classifications in this group included NC (four), CO (three), LC (one) and RH (one). Two cases had visible intrahepatic bacteria (E coli), which concurred with the results of bacterial culture. Three cases with positive hepatic cultures (one E fecium, one Staphylococcus, one E fecium + Staphylococcus + Clostridium) were FISH-negative.
Relationship of hepatic bacteria to concurrent disease
In 13 ILD with intrahepatic bacteria, all had concurrent non-hepatic disease. Intestinal disease, pancreatitis, or both intestinal disease and pancreatitis (three IBD: one mild and one severe LP enteritis, and one severe suppurative colitis), two intestinal lymphoma and five pancreatitis (one acute, three chronic and one chronic with carcinoma, with 2/13 cases having both IBD and pancreatitis) was present in 8/10 FISH-positive ILD cases in which intestinal and pancreatic biopsies were acquired. However, there was no significant difference in the prevalence of intrahepatic bacteria in cats with or without IBD, lymphoma or pancreatitis. Other concurrent diseases included carcinoma (two), diaphragmatic hernia, cystitis with cystic calculi and congenital portosystemic shunt.
Discussion
Inflammatory liver disease in cats is predominantly characterized by inflammation centered on hepatic bile ducts (cholangitis), rather than the parenchyma (hepatitis), and is widely considered to result from infection or aberrant immune responses. However, a clear understanding of the steps leading to ILD and its various phenotypes, such as neutrophilic and lymphocytic cholangitis, and specific involvement of bacteria and host immunity has yet to emerge. In this study we sought to determine the presence, type and location of intact bacteria within the livers of cats with various forms of ILD by use of FISH with oligonucleotide probes directed against bacterial rRNA. A subsidiary aim was to investigate the potential link between concurrent disease such as intestinal or pancreatic disorders, and the presence hepatic bacteria. We observed bacteria in sections from 21/39 (54%) cats with ILD, but in only 3/19 controls (P <0.05). The distribution of bacteria varied among the cases. In 13 ILD cases bacteria were visualized predominantly within portal vessels and venous sinusoids, or the hepatic parenchyma near the capsule. In only one cat, which had NC, were bacteria visible within bile ducts. In the remaining FISH-positive cases bacteria were restricted to the outer liver capsule and may represent contaminants. Bacterial cultures and FISH analysis were concordant in 15/23, and the predominant bacteria identified were common enteric forms, such as E coli and Enterococcus species. Concurrent pancreatic and intestinal disease was frequent in ILD cats, which, in concert with the intrahepatic distribution of enteric bacteria, suggests possible translocation across the intestinal tract as a likely route of infection.
FISH uses labeled oligonucleotide probes to detect the presence and spatial distribution of bacteria in a biological sample.11,13 The simultaneous application of probes labeled with different fluorochromes enables accurate distinction of intact bacteria from endogenous autofluorescence, for example EUB338-cy3 and non-EUB-338-6-FAM, and different types of bacteria within a mixed population, for example EUB338-cy3 and E coli-6-FAM. In this study we used a universal probe (EUB338 Cy-3) to determine the presence or absence of eubacteria, and followed up with specific probes, the selection of which was guided by the results of bacterial culture, bacterial morphology on eubacterial FISH and bacteria reported to be associated with feline ILD. We found that intact bacteria were significantly more common in liver sections from ILD than healthy controls. This difference could not be attributed to the method of sampling (surgical vs necropsy), as there was no significant difference in bacterial colonization of hepatic tissue samples obtained at surgery or necropsy in the control group. Our results are consistent with a study that identified bacteria (E coli and Streptococcus species) in samples from cats with LC obtained ante mortem, but not in non-inflamed controls sampled at necropsy. 14
The spatial distribution of bacteria in tissue sections could be divided into two distinct areas — intrahepatic and capsular. The relatively high proportion of samples with capsular bacteria (approximately 20% of ILD) may represent contamination during sampling, which has significant implications for culture- and PCR-based methods used to determine the presence or absence of bacteria in liver samples. In contrast to the similar prevalence of capsular bacteria in ILD and C, we observed a significantly higher prevalence of intrahepatic bacteria in ILD. Intrahepatic bacteria were located predominantly within portal vessels and venous sinusoids, or the hepatic parenchyma near the capsule, with bacteria observed within the bile duct of one case of NC with concurrent bile duct obstruction (BDO) and pancreatitis. This spatial distribution of bacteria within the livers of cats with ILD was not what we had anticipated. By assuming that ascending infection of the bile duct would be the most likely conduit for bacterial entry to the liver we expected to observe bacteria most frequently within the bile ducts. Instead, we observed intrahepatic bacterial colonization of venous sinuses, portal vessels and the hepatic parenchyma, suggesting the translocation of enteric bacteria across the mucosal barrier into the portal circulation as the more likely route for infection.
The overall prevalence of hepatic bacteria observed herein (54% of ILD, 33% intrahepatic), is substantially higher than the 11% (bacteria at any site) and 6% (intrahepatic) described in a recent study of feline cholangitis. 5 Because the FISH methodology employed in both studies is identical, this discordancy is likely owing to differences in the types of ILD examined by these studies, with the present study designed to incorporate a more diverse spectrum or ILD in contrast to the LC and hepatic lymphoma examined by Warren et al. 5 Direct comparison of the prevalence of intrahepatic bacteria in cats with LC and hepatic lymphoma in these two studies (present study 2/10 intrahepatic bacteria; Warren et al 5 study 2/54 intrahepatic bacteria) reveals no significant difference (P >0.05) in these subgroups between studies. Comparisons of different subgroups of ILD in the present study (LC, NC, AH, RH, CO) did not reveal significant differences in bacterial colonization; however, the number of cases we evaluated in each of these subgroups is relatively small and further studies are required to address this issue. To ascertain if specific histological features correspond with the presence of intrahepatic bacteria we correlated median numbers of neutrophils macrophages, plasma cells, lymphocytes and degree of periductular fibrosis in FISH-positive (intrahepatic bacteria) and FISH-negative samples. We found no significant correlation between these individual features and the presence of intrahepatic bacteria.
We did not identify a single primary bacterial species associated with ILD. Organisms identified by FISH were predominately enteric in origin, with E coli the most common, but E fecalis, S bovis and Clostridium species were also included. The highest density of bacterial colonization was observed with E coli in two cats with NC. One of these cats had micro-abscesses (see Figure 1), which was suspected as being sterile on routine histopathology. It is noteworthy that E coli has previously been associated with hepatic abscesses in cats. 15 The selection of species-specific FISH probes on the basis of bacterial morphology on eubacterial FISH, and the results of bacterial culture, restricted our analysis to cultivable bacteria and may have led us to underestimate the diversity of bacteria associated with feline ILD. The use of an unbiased culture-independent approach to generate an inventory of bacteria within the liver could have identified additional bacterial species with fastidious culture requirements. Thus, we may have missed difficult-to-culture bacteria, such as Bartonella species, which has been associated with mild LC/pericholangitis in 9/18 (50%) cats with experimental chronic infection by Bartonella henselae or Bartonella clarridgeiae. 16
We isolated bacteria from 11/23 cases in which culture was performed, and the bacterial species identified (five E coli, three Enterococcus, three Staphylococcus, one Actinomyces, one Streptococcus) are consistent with previous studies culturing bile or hepatic tissue from cats with ILD.6,7,12 The frequency of bacterial isolation in the present study (48%) is higher than the 14% and 36% previously reported for hepatic and biliary samples in cats, and this may reflect the use of wedge versus needle biopsies herein. 6 While FISH and culture results concurred in 15/23 cases (six positive, nine negative) there was no significant correlation between FISH and bacterial culture (Spearman’s rho 0.302, P >0.05). In eight cases where FISH did not correlate with culture, three FISH-positive samples had scant numbers of capsular bacteria. In five culture-positive, FISH-negative samples, two were positive for Staphylococcus species and one for Actinomyces species likely represent contaminants; however, FISH may have missed these bacteria because of the difficulty in permeabilizing these Gram-positive bacteria for analysis. In the remaining culture-positive FISH-negative samples (one Enterococcus species, one E coli) the reasons for discordancy are not apparent, but may reflect variation in the different samples used for culture and FISH. In practical terms it appears that FISH and bacterial culture have a complementary role in the evaluation of bacterial colonization in ILD. The isolation of cultivable bacteria enables an antimicrobial susceptibility profile to be generated to inform treatment, whereas FISH analysis can identify a true bacterial presence without associated problems of culture contamination.
Feline ILD, in particular cholangitis, is reported to occur commonly with either intestinal and/or pancreatitic disease. The term ‘feline triaditis’ has been coined to reflect the association of these three entities when they all occur together.9,17 Hence, a subsidiary aim of this study was to investigate a possible link between concurrent diseases, such as intestinal or pancreatic disorders, associated with ILD and the presence hepatic bacteria. Concurrent non-hepatic disease, predominantly pancreatic and intestinal, was present in all 13 cats with intrahepatic bacteria. In this series 8/10 cats biopsied had evidence of pancreatitis, IBD or GI lymphoma, suggesting there could be a link between GI or pancreatic disease with ILD. Three cats with IBD were classified as mild in one and severe in two. Also, concurrent chronic pancreatitis and IBD was observed in two cases of ILD and intrahepatic bacteria (NC and LC). Interestingly, the highest incidence of hepatic bacterial colonization was observed in cats with RH (5/12) and AH (2/2), where inflammation is associated with hepatocellular and periportal inflammation. In the RH group histological changes were considered to be secondary (reactive) to a primary non-hepatic condition, most frequently GI lymphoma (four), pancreatitis (three) and structural GI disease (diaphragmatic hernia, string foreign body). There were only two cases of AH, one of which had pancreatitis and diabetes mellitus. Taken as a whole, these results suggest that concurrent non-hepatic disease could promote hematogenous seeding of the liver with enteric bacteria. 4 This notion is supported by studies in a feline model of pancreatitis, which demonstrated hematogenous and transmural spread of E coli from the colon to the liver and pancreas. 18
Conclusions
This study represents the first investigation to determine the presence of intact bacteria in the livers of cats that encompass the broad spectrum of feline ILD using culture-independent methodology. We observed intrahepatic bacteria in 33% of 39 cats with ILD compared with 5% of 19 controls, and the highest bacterial numbers in cats with E coli-associated NC. Our findings suggest that bacterial contamination during hepatic sampling should be a concern, and that FISH and bacterial culture have a complementary role by enabling accurate diagnosis of infection and targeted antimicrobial therapy. The type of intrahepatic bacteria, their spatial distribution within the liver, and the high prevalence of concurrent diseases that reduce intestinal barrier function suggests possible enteric translocation or hematogenous seeding as the potential source of infection. Further studies are required to determine the relationship of intrahepatic bacteria to individual subgroups of ILD.
Acknowledgments
We thank Francis Davis and Pat Fisher for technical support.
Footnotes
This work was presented as an abstract at the 2009 ACVIM Forum, Montreal, Canada (JVIM 2009; 23: 729)
Funding: Funding was provided by the Companion Animal Foundation, Colorado State University and The International Feline Foundation.
The authors do not have any potential conflicts of interest to declare.
Accepted: 26 June 2013
References
- 1. Gagne JM, Weiss DJ, Armstrong PJ. Histopathologic evaluation of feline inflammatory liver disease. Vet Pathol 1996; 33: 521–526. [DOI] [PubMed] [Google Scholar]
- 2. Day DG. Feline cholangiohepatitis complex. Vet Clin North Am Small Anim Pract 1995; 25: 375–385. [DOI] [PubMed] [Google Scholar]
- 3. Van den Ingh TS, Cullen JM, Twedt DC, et al. Morphological classification of biliary disorders of the canine and feline liver. In: Rothuizen J, Bunch SE, Charles JE, et al. (eds). WSAVA standards for clinical and histological diagnosis of canine and feline liver diseases. Philadelphia, PA: Elsevier, 2006, pp 68–71. [Google Scholar]
- 4. Greiter-Wilke A, Scanziani E, Soldati S, et al. Association of Helicobacter with cholangiohepatitis in cats. J Vet Intern Med 2006; 20: 822–827. [DOI] [PubMed] [Google Scholar]
- 5. Warren A, Center S, McDonough S, et al. Histopathologic features, immunophenotyping, clonality, and eubacterial fluorescence in situ hybridization in cats with lymphocytic cholangitis/cholangiohepatitis. Vet Pathol 2011; 48: 627–641. [DOI] [PubMed] [Google Scholar]
- 6. Wagner KA, Hartmann FA, Trepanier LA. Bacterial culture results from liver, gallbladder, or bile in 248 dogs and cats evaluated for hepatobiliary disease: 1998–2003. J Vet Intern Med 2007; 21: 417–424. [DOI] [PubMed] [Google Scholar]
- 7. Brain PH, Barrs VR, Martin P, et al. Feline cholecystitis and acute neutrophilic cholangitis: clinical findings, bacterial isolates and response to treatment in six cases. J Feline Med Surg 2006; 8: 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boomkens SY, Kusters JG, Hoffmann G, et al. Detection of Helicobacter pylori in bile of cats. FEMS Immunol Med Microbiol 2004; 42: 307–311. [DOI] [PubMed] [Google Scholar]
- 9. Weiss DJ, Gagne JM, Armstrong PJ. Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis, and nephritis in cats. J Am Vet Med Assoc 1996; 209: 1114–1116. [PubMed] [Google Scholar]
- 10. Van den Ingh TS, Van Winkle T, Cullen JM, et al. Morphological classification of parenchymal disorders of the canine and feline liver. In: Rothuizen J, Bunch SE, Charles JE, et al. (eds). WSAVA standards for clinical and histological diagnosis of canine and feline liver diseases. Philadelphia, PA: Elsevier, 2006, pp 85–101. [Google Scholar]
- 11. Janeczko S, Atwater D, Bogel E, et al. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet Microbiol 2008; 128: 178–193. [DOI] [PubMed] [Google Scholar]
- 12. Priestnall SL, Wiinberg B, Spohr A, et al. Evaluation of ‘Helicobacter heilmannii’ subtypes in the gastric mucosas of cats and dogs. J Clin Microbiol 2004; 42: 2144–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amann RI, Krumholz L, Stahl DA. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol 1990; 172: 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Otte CM, Gutiérrez OP, Favier RP, et al. Detection of bacterial DNA in bile of cats with lymphocytic cholangitis. Vet Microbiol 2012; 156: 217–221. [DOI] [PubMed] [Google Scholar]
- 15. Sergeeff JS, Armstrong PJ, Bunch SE. Hepatic abscesses in cats: 14 cases (1985–2002). J Vet Intern Med 2004; 18: 295–300. [DOI] [PubMed] [Google Scholar]
- 16. Kordick DL, Brown TT, Shin K, et al. Clinical and pathologic evaluation of chronic Bartonella henselae or Bartonella clarridgeiae infection in cats. J Clin Microbiol 1999; 37: 1536–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Twedt DC, Armstrong PJ. Feline inflammatory liver disease. In: Bonagura JD, Twedt DC, (eds). Current veterinary therapy XIV. St Louis, IL: Elsevier, 2009, pp 576–581. [Google Scholar]
- 18. Widdison AL, Karanjia ND, Reber HA. Routes of spread of pathogens into the pancreas in a feline model of acute pancreatitis. Gut 1994; 35; 1306–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]