Abstract
Many feline breeds have been generated from a small number of ancestors. Thus, breed-specific peculiarities can be expected, which could include haematological and biochemical measurements. Despite this, there are only a few reports on breed-specific reference intervals (RI). This information is essential in routine practice where results from individual patients are usually compared with an RI. The aim was to compare haematological and biochemical data from clinically healthy Abyssinian, Holy Birman, Norwegian Forest and Siberian cats with published RIs to assess whether the published RIs are acceptable in these breeds. Comparison with established RIs using guidelines from the National Committee for Clinical Laboratory Standards and the American Society of Veterinary Clinical Pathology, revealed a number of breed-related clinicopathological differences. New RIs were established, but in most cases the new RIs overlapped with published RIs, and the use of the breed-specific data would minimally affect the clinical interpretation of laboratory results. Important differences that could result in misinterpretation of laboratory results were as follows: microcytosis and high α2-globulin concentrations in Abyssinian cats; high serum creatinine, α2-globulin and glucose concentrations in Holy Birman cats; high serum alkaline phosphatase activity and calcium and phosphate concentration in Norwegian Forest cats; low β2-globulin and γ-globulin concentrations in Norwegian Forest and Siberian cats. Breed-specific RIs should be used for these analytes. In addition, care should be taken in interpreting clinicopathological data in purebred cats for which specific RIs have not been established.
Introduction
In routine practice, interpretation of laboratory data from individual patients is based on comparison with reference intervals (RIs). RIs are representative when the population they refer to is composed of healthy subjects. 1 This usually occurs when the reference population is wide enough to minimise the effect of inter- and intra-individual variability or of analytical variability. 2 This is not necessarily the case in veterinary medicine, as it can be difficult to sample large numbers of animals, and multiple pre-analytical factors may influence the RIs. The National Committee for Clinical Laboratory Standards (NCCLS) and the American Society of Veterinary Clinical Pathology (ASVCP) released guidelines to determine RIs on small-sized samples.3,4 The use of population-based RIs has recently been questioned, and it has been proposed that they only be used for analytes with a high ‘index of individuality’ (ie, the ratio of intra- to inter-individual biological variation). 5 Conversely, subject-based RIs are recommended when the index of individuality is low. However, this approach also has limitations, as there are cost and owner compliance issues. As a compromise, RIs specific for subgroups of animals with similar physiological features may be used. 1 Therefore, breed-specific RIs may be recommended for breeds that have peculiar metabolic or physiological patterns. These patterns may arise from the adaptation to environmental conditions, to a specific aptitude (ie, racing horses or dogs) or to the ‘additive’ effect of the generation of new breeds from pre-existing breeds. 6
In dogs, many breed-specific peculiarities have been described (eg, macrothrombocytopenia in Cavalier King Charles Spaniels, 7 microcytosis in Japanese dogs, 8 high serum concentration of bile acids in Maltese dogs 9 and several clinicopathological peculiarities in Greyhounds 10 ). Theoretically, breed-specific peculiarities should occur more frequently in cats, where many breeds were generated from a small number of ancestors, and breed initiation occurred more recently than in dogs. 11 This has resulted in a high incidence of heritable disease (eg, polycystic kidney disease in Persian cats, hypertrophic cardiomyopathy in Norwegian Forest and Siberian cats, amyloidosis in Abyssinian and Siamese/Oriental cats, pyruvate kinase deficiency in several breeds). 12 It is thus possible that breed-specific haematological or biochemical difference occur, but only a few reports on breed-specific feline RIs are available. 13
The aim of this study was to compare haematological and biochemical data from clinically healthy cats of feline breeds (Abyssinian, Holy Birman, Norwegian Forest, Siberian) with published feline RIs to determine whether published RIs can be validated or rejected in these breeds, and to establish breed-specific RIs.
Materials and methods
Selection of cases and samplings
This study used blood samples collected from intact clinically healthy cats aged 1–9 years. Details of the study population are summarised in Table 1. Samples were from a research project on Holy Birman cats funded by the Winn Feline Foundation or submitted by veterinarians involved in plans of health monitoring through periodical wellness visits promoted by breed associations. Based on our regulations, formal ethical approval was not needed, as cats were sampled with the informed consent of the owners during routine check-up visits.
Table 1.
Summary of the demographic information of cats included in the study
| Breed | Number of catteries | Number of cats per cattery | Number of cats |
Age range min–max (median) |
||
|---|---|---|---|---|---|---|
| Total | M | F | ||||
| Abyssinian | 8 | 3–8 | 45 | 17 | 28 | 1–8 (3) |
| Holy Birman | 23 | 2–5 | 91 | 44 | 47 | 1–9 (4) |
| Norwegian Forest | 11 | 2–6 | 50 | 22 | 28 | 1–9 (4) |
| Siberian | 6 | 4–14 | 47 | 23 | 24 | 1–6 (2) |
M = male; F = female; Min = minimum; Max = maximum
Strict inclusion and exclusion criteria were fixed a priori as recommended by NCCLS. 3 Specifically, the inclusion criteria were as follows:
Cats living in single- or multicat households, receiving a normal diet based on commercially-available feline food, regularly submitted to veterinary visits and periodically tested for feline immunodeficiency virus [enzyme-linked immunosorbent assay (ELISA) test], feline leukaemia virus (ELISA test), feline coronavirus (FCoV) (anti-FCoV serology by indirect immunofluorescence test);
No recent history of diseases or of pathophysiological conditions potentially affecting blood results (eg, tumours, severe infectious diseases, oestrus, pregnancy, lactation, etc);
Regular worming and vaccination;
No medications administered in the 3 months before sampling;
No clinical signs before sampling or in the 3 months following.
Exclusion criteria were as follows:
Cats that did not fulfil the inclusion criteria;
Cats younger than 1 year were excluded as several pre-analytical factors (growth, frequency of vaccinations, etc) can affect haematological and biochemical variables;
Samples characterised by lipaemia, haemolysis or icterus.
Blood (approximately 3 ml) was collected from animals fasted at least for 12 h and transferred into plain tubes and/or into glass tubes containing K3-ethylenediamine tetra-acetic acid (EDTA) (Venoject, Terumo Italia). When possible, both the tubes were filled to their maximum capacity. When the blood volume was low, only one tube was filled by the referring veterinarian.
Samples were submitted to the laboratory, stored at 4°C and processed within 12 h. Anti-coagulated tubes were immediately used to perform routine haematology. Plain tubes were centrifuged (1800 g for 8 min) to obtain serum, to be stored at −20°C until biochemical analyses, performed within 1 month of collection.
Haematology
Haematology was performed using a laser haematology analyser (Sysmex XT-2000iV; Sysmex), validated for feline blood, 14 equipped with a multispecies software. The parameters listed in Table 2 were recorded. Sysmex XT-2000iV applies two different technologies for the platelet (PLT) counts: one based on impedance channel of the instrument, potentially affected by PLT clumps and PLT activation; and the other based on laser technology (less affected by PLT clumping). Based on the frequency of pre-analytical artifacts observed in impedance counts of feline blood, 15 only the results of laser counts were included in this study to avoid spurious thrombocytopenias. Quality control and calibration were periodically performed with e-check Xe (Sysmex). The differential leukocyte count and the PLT estimate were evaluated on May-Grünwald Giemsa-stained smears. The PLT estimate was considered adequate when at least eight PLTs per high power field (HPF) were present. PLT clumping was scored as suggested in a previous study: 16 samples with a score >2 (aggregates of 2–4 PLTs calculated on the mean of the 10 values observed on HPF randomly selected at the edge and tail of the film) were excluded from the establishment of RIs, independently of the result reported by the instrument.
Table 2.
Summary of the number of samples available for each analyte in the four breeds included in this study
| Abyssinian | Holy Birman | Norwegian Forest | Siberian | |
|---|---|---|---|---|
| Routine haematology (RBC count, Hb, Ht, MCV, MCH, MCHC, WBC, differential leukocyte count) | 45 | 84 | 42 | 44 |
| Reticulocyte percentage | 44 | 73 | 30 | 44 |
| Platelets (optical count and clump score <2) | 42 | 44 | 27 | 32 |
| Serum protein electrophoresis | 36 | 71 | 28 | 43 |
| Total proteins (biuret method) | 44 | 80 | 28 | 43 |
| Glucose (GOD–POD method) | 44 | 60 | 28 | 38 |
| Urea (urease method) | 44 | 81 | 28 | 47 |
| Creatinine (Jaffé method) | 44 | 81 | 29 | 44 |
| Alanine aminotransferase (kinetic IFCC method) | 42 | 79 | 28 | 42 |
| Alkaline phosphatase (kinetic IFCC method) | 44 | 67 | 28 | 41 |
| Cholesterol (cholesterol oxidase method) | 44 | 51 | 26 | 44 |
| Triglycerides (lipase–glycerol kinase method) | 40 | 75 | 29 | 35 |
| Calcium (orthocresoftaleine method) | 35 | 43 | 24 | 34 |
| Phosphate (phosphomolibdate method) | 42 | 67 | 24 | 34 |
| γ-glutamyl transferase (kinetic IFCC method) | 36 | 67 | 22 | 39 |
RBC = red blood cells; Hb = haemoglobin; Ht = haematocrit; MCV = mean cell volume; MCH = mean cell haemoglobin; MCHC = mean cell haemoglobin concentration; WBC = white blood cells; GOD–POD = glucose oxidase peroxidase; IFCC = International Federation of Clinical Chemistry
Clinical chemistry
Biochemistry was performed using an automated spectrophotometer (Cobas Mira; Roche) with reagents provided by Real Time. The analytes listed in Table 2 were measured. When the volume of serum was low, not all biochemical tests were performed (see Table 2).
Agarose gel electrophoresis was performed using an automated analyser (Hydrasis; Sebia Italia) and the manufacturer’s reagent kit (Hydragel β1-β2; Sebia). After migration (7 mins, 800 V), gels were stained with amidoschwarz, destained, dried, scanned and densitometrically analysed using specific software (Phoresis; Sebia). Absolute protein concentrations (g/l) for each electrophoretic fraction were calculated based on total serum protein and on the percentage of the area under each peak.
Comparison with published feline RIs
For each analyte, results obtained in each breed were compared with RIs published in veterinary textbooks17,18 using specific software (Analyse-it v 2.21) after removal of data that, according to the Tukey rule, behave as outliers interpretable as aberrant observations. 3
The comparison with RIs was based on the following steps:
Data were ordered using the RAND function of Microsoft Excel
The first 20 items were selected and compared with the published RIs
Published RIs were validated if <10% of data (n = 2) was outside the published RI
Published RIs were rejected if >25% of data (n = 5) was outside the RI
If 10–25% of data (n = 3 or n = 4) were outside the published RIs, the next 20 items in the list were selected and compared with the RIs as described above, using the threshold of 10% of data outside the RIs (n = 2) to validate or reject the RIs.
Establishment of new breed-specific RIs
A breed-specific RI was generated using an Excel spreadsheet with the Reference Value Advisor (v 2.0) set of macroinstructions. 19 The software performs the following computations recommended by the International Federation of Clinical Chemistry-Clinical and Laboratory Standards Institute: 3 descriptive statistics (eg, mean, median, SD, minimum and maximum values); tests of normality (Anderson–Darling with histograms and Q–Q plots and Box–Cox transformation); outlier analysis. Both Dixon–Reed and Tukey tests were used, and outliers classified as ‘suspected’ were retained, as recommended by the ASVCP guidelines, 4 while far outliers were removed from the analysis. RIs were calculated using standard and robust methods on both non-transformed and transformed data. The software indicates the best method to define the RI based on data distribution. A non-parametric bootstrap method was used to calculate the 90% confidence interval.
Partitioning of RIs
For each analyte and within each breed, results from male and female cats were compared using the Mann–Whitney U-test and results of young cats (<3 years), adult cats (3–6 years) and old cats (>6 years) using a Kruskall–Wallis test with Bonferroni correction.
Results
Comparison with published RIs and establishment of new RIs
The distribution of data recorded for each analyte is displayed in Figures 1-3. The percentage of observations falling outside the published RIs is reported in Table 3, which also includes the new RIs generated in this study. According to the ASVCP guidelines, 4 details of the new RIs are also reported (see Supplementary Material). As shown in the Supplementary Material, several published RIs were rejected and new RIs were established. Specifically, the following species-specific RIs were determined.
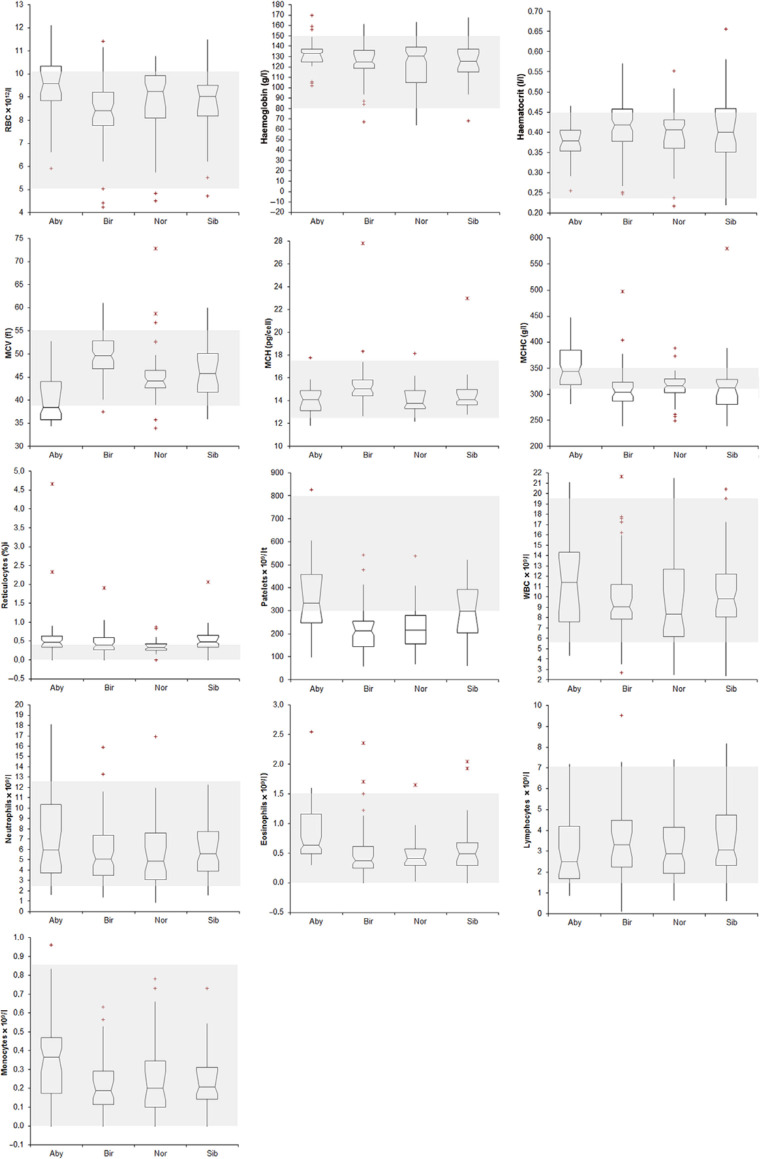
Figure 1.
Haematological results recorded in the four breeds of cats included in this study [Abyssinian (Aby), Holy Birman (Bir), Norwegian Forest (Nor) and Siberian (Sib)]. The boxes indicates the I–II interquartile range (IQR), the horizontal line indicates the median values, whiskers extend to further observation within quartile I minus 1.5 × IQR or to further observation within quartile III plus 1.5 × IQR. ‘+’ indicates near outliers (ie, values exceeding quartiles I or III minus or plus 1.5 × IQR); the asterisks indicate far outliers (ie, values exceeding quartiles I or III minus or plus 1.5 × IQR). The grey area indicates the reference intervals reported in literature for feline haematological data. 18 Graphs corresponding to basophils and band neutrophils counts are not included in this figure. RBC = red blood cells; MCV = mean cell volume; MCH = mean cell haemoglobin; MCHC = mean cell haemoglobin concentration; WBC = white blood cells
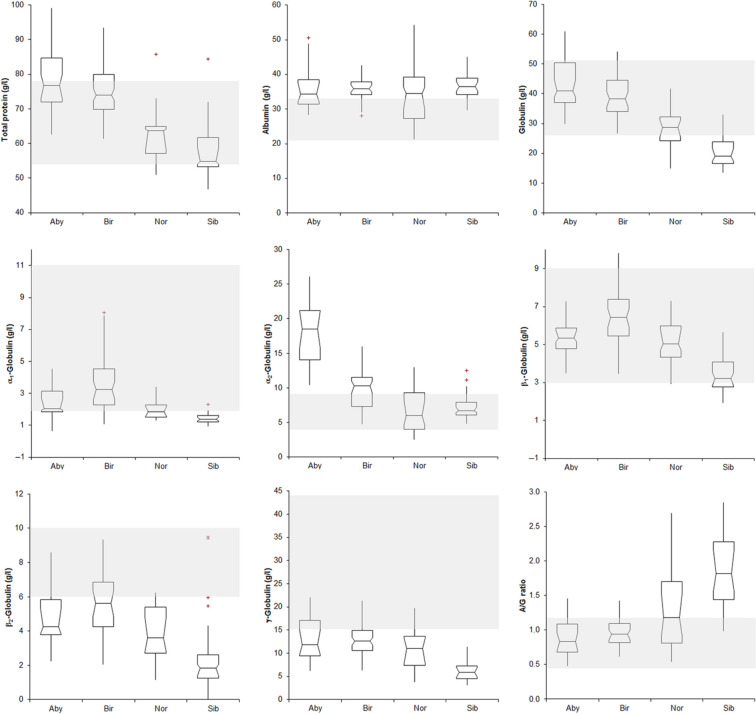
Figure 2.
Results of electrophoretograms in the four breeds of cats included in this study [Abyssinian (Aby), Holy Birman (Bir), Norwegian Forest (Nor) and Siberian (Sib)]. See Figure 1 for interpretation of box and whisker plots. The grey area indicates the reference intervals reported in the literature for feline biochemical data. 17 A/G ratio = albumin/globulin ratio
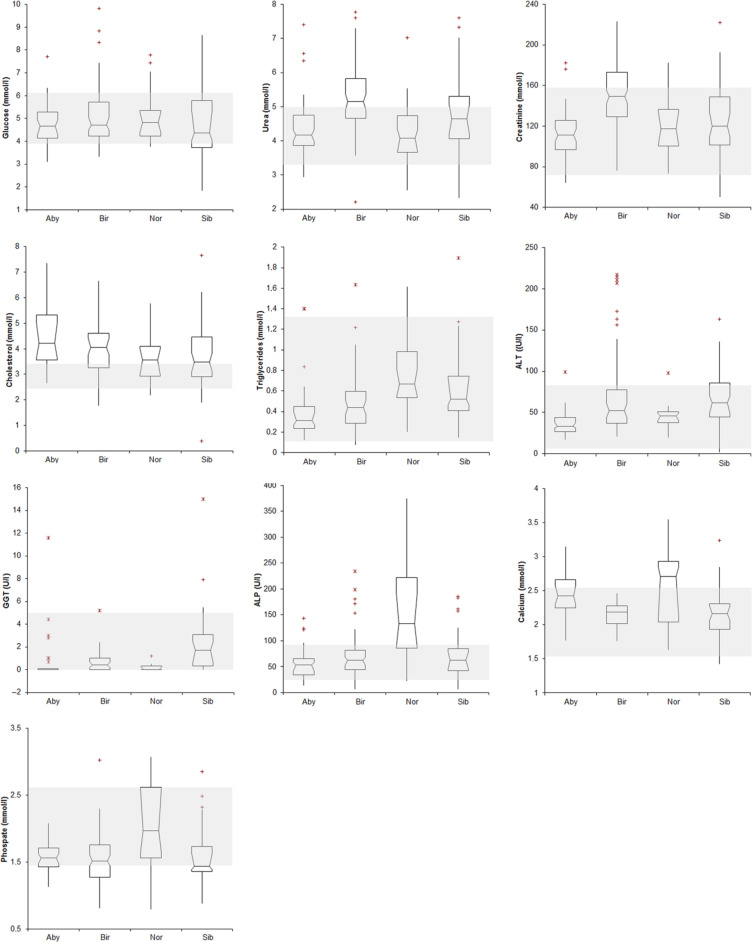
Figure 3.
Biochemical results recorded in the four breeds of cats included in this study [Abyssinian (Aby), Holy Birman (Bir), Norwegian Forest (Nor) and Siberian (Sib)]. See Figure 1 for interpretation of box and whisker plots. The grey area indicates the reference intervals reported in the literature for feline biochemical data. 17 ALT = alanine aminotransferase; GGT = γ-glutamyl transferase; ALP = alkaline phosphatase
Table 3.
Published reference intervals (RIs) and the new breed-specific RIs. For each breed, the percentage of 20 randomly selected observations falling outside the published RI is reported. Published RIs were rejected when the percentage was >25% at first analysis or >10% in two consecutive analyses of the dataset. When the published RI was rejected, a new RI, reported in bold, was established following the National Committee for Clinical Laboratory Standards 3 and American Society of Veterinary Clinical Pathology 4 guidelines. Statistical details of the new RIs are reported in the Supplementary Material (Tables S1–S4)
| Published RI |
Abyssinian |
Holy Birman |
Norwegian Forest |
Siberian |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Outside RI (%) |
Breed RI | Outside RI (%) |
Breed RI | Outside RI (%) |
Breed RI | Outside RI (%) |
Breed RI | ||
| RBC × 1012/l | 5–10 18 | 40 | 6.0–12.1 | 5 | nn | 25 (30) | 4.5–10.8 | 25 (5) | nn |
| Haemoglobin (g/l) | 80–150 18 | 5 | nn | 0 | nn | 5 (20) | nn | 20 (0) | nn |
| Haematocrit (l/l) | 0.24–0.45 18 | 5 | nn | 30 | 0.28–0.55 | 30 | 0.22–0.55 | 35 | 0.23–0.65 |
| MCV (fl) | 39–55 18 | 60 | 34–53 | 15 (25) | 40–59 | 15 (10) | nn | 25 (25) | 36–60 |
| MCH (pg/cell) | 12.5–17.5 18 | 5 (25) | nn | 5 | nn | 5 | nn | 0 | nn |
| MCHC (g/l) | 310–350 18 | 45 | 288–468 | 60 | 240–400 | 40 | 249–389 | 70 | 239–559 |
| Reticulocyte (%) | <0.4 18 | 0 | nn | 25 (30) | <1.2 | 20 (30) | 0.1–0.8 | 40 | <1.9 |
| Leukocytes × 109/l | 5.5–19.5 18 | 0 | nn | 0 | nn | 10 | nn | 5 | nn |
| Neutrophils × 109/l | 2.5–12.5 18 | 5 | nn | 10 | nn | 10 | nn | 5 | nn |
| Basophils × 109/l | 0 18 | 0 | nn | 0 | nn | 0 | nn | 0 | nn |
| Band neutrophils × 109/l | <0.3 18 | 0 | nn | 0 | nn | 0 | nn | 0 | nn |
| Eosinophils × 109/l | <1.5 18 | 5 | nn | 0 | nn | 5 | nn | 0 | nn |
| Lymphocytes × 109/l | 1/.5–7.0 18 | 15 (15) | 0.9–8.9 | 5 (25) | nn | 30 (10) | nn | 10 | nn |
| Monocytes × 109/l | <0.85 18 | 0 | nn | 0 | nn | 0 | nn | 0 | nn |
| Platelets × 109/l | 300–800 18 | 15 (20) | 125–754 | 40 | 61–534 | 50 | 72–603 | 30 | 42–572 |
| Total protein (g/l) | 54–78 17 | 40 | 63–98 | 15 (35) | 62–88 | 10 | nn | 35 | 47–83 |
| Albumin (g/l) | 21–33 17 | 60 | 27–54 | 90 | 29–42 | 55 | 19–55 | 95 | 30–45 |
| Total globulin (g/l) | 26–51 17 | 20 (21) | 30–65 | 10 | nn | 50 | 16–41 | 95 | 14–40 |
| A/G ratio | 0.45–1.19 17 | 15 (29) | 0.44–1.45 | 15 (10) | nn | 55 | 0.47–2.67 | 95 | 1.00–2.84 |
| α1-Globulin (g/l) | 2–11 17 | 60 | 0–5 | 30 | 1–8 | 55 | 1–3 | 100 | 1–3 |
| α2-Globulin (g/l) | 4–9 17 | 100 | 10–27 | 60 | 5–16 | 65 | 1–14 | 0 | nn |
| β1-Globulin (g/l) | 3–9 17 | 0 | nn | 5 | nn | 0 | nn | 55 | 2–7 |
| β2-Globulin (g/l) | 6–10 17 | 70 | 2–10 | 70 | 2–9 | 95 | 1–7 | 100 | 1–9 |
| γ-Globulin (g/l) | 17–44 17 | 75 | 6–23 | 90 | 8–24 | 95 | 3–21 | 100 | 3–13 |
| Glucose (mmol/l) | 3.89–6.11 17 | 20 (25) | 3.16–7.54 | 35 | 3.39–9.30 | 5 | nn | 30 (25) | 2.03–8.04 |
| Urea (mmol/l) | 3.33–5.00 17 | 30 | 2.95–7.29 | 45 | 3.57–7.59 | 30 | 2.77–7.25 | 60 | 2.35–7.55 |
| Creatinine (mmol/l) | 70.7–159.1 17 | 15 (0) | nn | 40 | 97.2–221.0 | 0 | nn | 15 (30) | 53.0–221.0 |
| Cholesterol (mg/dl) | 2.46–3.37 17 | 75 | 2.69–7.32 | 72 | 1.45–6.52 | 60 | 2.25–6.62 | 35 | 02.58–7.48 |
| Triglycerides (mg/dl) | 0.11–1.29 17 | 5 | nn | 5 | nn | 0 | nn | 5 | nn |
| ALT (U/l) | 6–83 17 | 0 | nn | 35 | 23–120 | 5 | nn | 35 | 2–161 |
| GGT (U/l) | <5.1 17 | 0 | nn | 0 | nn | 0 | nn | 5 | nn |
| ALP (U/l) | 25–93 17 | 20 (35) | 14–143 | 10 | nn | 70 | 22–505 | 30 | 8–186 |
| Phosphate (mmol/l) | 1.45–2.62 17 | 25 (35) | 1.13–2.07 | 5 (25) | nn | 50 | 0.74–3.55 | 55 | 2.03–3.81 |
| Calcium (mg/dl) | 1.55–2.55 17 | 35 | 1.89–3.09 | 0 | nn | 45 | 1.36–3.84 | 20 | 1.59-2.95 |
RBC = red blood cells; MCV = mean cell volume; MCH = mean cell haemoglobin; MCHC = mean cell haemoglobin concentration; A/G ratio: albumin/globulin ratio; ALT = alanine aminotransferase; GGT = γ-glutamyl transferase; ALP = alkaline phosphatase; nn = breed-specific RI not needed
Abyssinian cats
New RI were established for 19 analytes: red blood cell count (RBC); total protein; albumin; total and α2-globulin; cholesterol; calcium (higher reference limit); mean corpuscular volume (MCV); PLT; α1-, β2-and γ-globulin; phosphate (lower reference limit); mean corpuscular haemoglobin concentration (MCHC); lymphocytes; albumin/globulin (A/G) ratio; glucose; urea; alkaline phosphatase (ALP) (wider RI).
Holy Birman cats
New RIs were established for 16 analytes: haematocrit (Ht); MCV; reticulocytes; total protein; albumin; α2-globulins; urea; creatinine; alanine aminotransferase (ALT) (higher reference limit); PLT; α1-, β2- and γ-globulin (lower reference limit); MCHC; glucose; cholesterol (wider RI).
Norwegian Forest cats
New RIs were established for 17 analytes: reticulocytes; A/G ratio (higher reference limit); PLT; total-, α1-, β2- and γ−globulin (lower reference limit); RBC; Ht; MCHC; albumin; α2 globulin; urea; cholesterol; ALP; phosphate; calcium (wider RI).
Siberian cats
New RIs were established for 21 analytes: reticulocytes; albumin; A/G ratio; phosphate; calcium (higher reference limit); PLT; total-, α1-, β1, β2 and γ−globulin (lower reference limit); Ht; MCV; MCHC; total protein; glucose; urea; creatinine; cholesterol; ALT; ALP (wider RI).
Partitioning based on sex and age
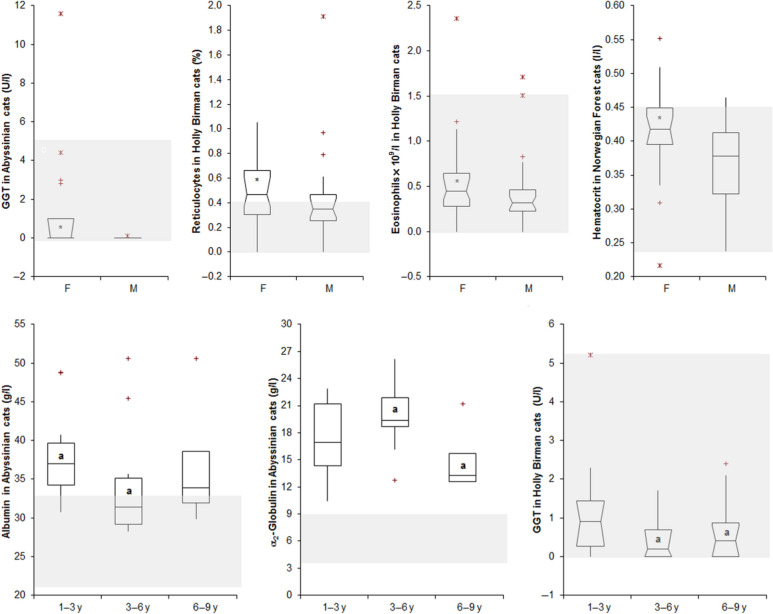
The few significant sex- or age-related differences recorded are displayed in Figure 4. In all these cases, however, median, minimum and maximum values recorded in the different groups were very similar to each other.
Figure 4.
Haematological or biochemical results that were signifcantly different in female (F) and male (M) cats, or in cats grouped according to age range (<3 years, 3–6 years, >6 years). See Figure 1 for the interpretation of box and whiskers plots. The black bolded asterisk within the boxes indicates a significant gender-related difference. In the comparison of age groups, boxes labelled with the same letters (‘a’) are significantly different to each other. GGT = γ-glutamyl transferase
Discussion
This study demonstrated breed-specific peculiarities in four feline breeds. It is unlikely that this was a result of environmental factors or composition of groups, as all the cats were housed in similar environments and received balanced commercial diets, and the age distribution and numbers of male and female did not differ between the breeds. In addition, partitioning revealed only a few significant age- or sex-related differences that were not clinically relevant, as results of different groups were very similar to each other. With respect to partitioning regarding age, the lack of significant differences may be owing to the fact that the three age groups are not biologically different to each other. Age-related peculiarities usually occur in kittens or in cats older than 10 years and neither were present in our caseload.
Theoretically, RIs generated ‘in house’ from a reference population of healthy individuals using the same instruments and methods of validation as those in this study should have been used for comparison;2–4 however, other studies have also used previously established textbooks ranges. 20 We preferred to follow this approach as, with a few exceptions, RIs from textbooks are usually very similar to those generated by any laboratory from the general hospital population, unless some patient peculiarity (eg, breed, age range, aptitude) exists. Nevertheless, this approach may have induced some of the observed discrepancies because the reference population may be different from that of this study (eg, our RIs have been generated from intact cats, younger than 9 years, while the general hospital population usually comprises neutered and/or aged animals), or because different test methods or instruments have been used. The latter information is rarely detailed in textbooks but it is likely that different, and possibly older, methodologies have been used. This is clearly seen in Figures 1–3, where in some cases it is evident that values were outside the RIs reported in textbooks for all four breeds. This is likely the case for reticulocyte percentages, as laser-based analysers are more analytically sensitive than manual counts and provide higher percentages than those reported in textbooks. 21 It may also be true for electrophoretic fractions, which, in the current study, were often lower than the reference RIs in all the breeds (eg, α1, β2 and γ-globulins), as a previous study showed that RIs reported in older textbooks were probably not adequate. 22 Similarly, discrepancies between measured urea, cholesterol and phosphate concentrations and RIs could have been owing to different analytical or statistical methods.
Automated PLT counts in cats may be falsely low owing to PLT clumps, unless anticoagulants, such as citrate, are used, or prostaglandin is added to the sample.23,24 Samples in this study were collected in EDTA: to minimise the influence of falsely low PLT count, therefore, samples with evident clumping, scored as proposed in another study, 16 were excluded. Nevertheless, individual cats still had low PLT counts despite an adequate PLT estimate. PLT estimates have been shown to be more reliable than automated counting; 25 therefore, the RIs determined in the study will underestimate PLT counts in the breeds.
The RIs reported in the literature for the other haematological parameters are so wide, possibly reflecting a more varied population being used to establish the textbook RIs, that most of the values recorded in the present study fell within published RIs. In some cases, the distribution of data reported in Figure 1 shows evidence of a narrower range than RIs from textbooks. This sometimes occurred for all the breeds (eg, mean corpuscular haemoglobin), but it was occasionally seen for a specific parameter in a single breed (eg, MCV of Norwegian Forest cat). In our opinion, this is a limitation of the transference methods for RIs, which allows classification of differences from the claimed RI only if one, or both, observations is outside the reference limits.
The breeds included in this study were selected for two main reasons. First, collaboration with breeders and breeding associations allowed us to collect a sufficient number of samples to apply strict inclusion criteria and have enough residual data to establish RIs as recommended;2–4 second, RIs are needed in these breeds, as three of them are prone to diseases that need veterinary visits and eventual blood testing (amyloidosis for Abyssinian cats, feline infectious peritonitis for Holy Birman cats, hypertrophic cardiopathy for Norwegian Forest cats). 6 Siberian cats have recently become popular; thus, the rate of inbreeding is likely to have increased. Breed-specific haematological RIs have not been investigated in these four breeds. Breed-specific biochemical RIs have previously been determined for Holy Birman cats. 13
The validation of published RIs revealed that for a variable proportion of analytes, breed-specific RIs should be designed. In most cases, however, the difference between published and new RIs was minimal (ie the reference limits of the two RIs were very close to each other) and likely due to the limitations of the RIs published in textbooks, as mentioned previously. In this case, it would be more correct to use the new RIs, but the use of published RIs would minimally affect clinical decisions. Conversely, if one or both limits of the new RI are very different from the published RIs and only a partial overlapping exists, a new RI would be recommended.
It is worth discussing some of the breed peculiarities in more detail. Abyssinian cats tend to have microcytic RBC. In other species, this may depend on iron deficiency. 8 Iron concentrations and markers of iron deficiency were not measured in this study, but no signs of iron deficiency were recorded. Microcytosis occurs in canine breeds with abnormal RBC membrane pumps 26 or in humans with peculiar haemoglobin phenotypes. 27 This latter hypothesis may also apply to cats, as they have a polymorphic β-globin system; 28 this merits further investigation. Abyssinian cats also had surprisingly high concentrations of α2-globulins and cholesterol. The former may suggest a ‘hyper-reactive’ innate response, even in the absence of clinically evident inflammation. This hypothesis is supported by an observation (SP) of severe increases of acute phase proteins (APP), including serum amyloid A (SAA), in Abyssinian cats with different diseases. In turn, this ‘hyper-reactivity’ might justify the predisposition of this breed to systemic amyloidosis, which is sustained by the increased SAA observed in Abyssinian cats.29,30 The trend to hypercholesterolaemia may also be associated with the predisposition to amyloidosis, as SAA interacts with cholesterol-rich, high-density lipoproteins. 31 However, the upper RI of cholesterol in all four breeds is increased, so the apparent increase in cholesterol could just reflect different test methodology from the textbook RI. Nonetheless, the association of high α2-globulins, cholesterol and amyloidosis merits investigation in future studies.
Holy Birman cats did not have haematological peculiarities. Conversely, biochemistry confirmed the high concentration of creatinine and urea reported in previous studies.13,32 The high concentration of urea may depend on the inadequacy of RIs published in textbooks. High serum creatinine concentrations, attributed to increased muscle mass has been reported in Greyhounds. 10 Increased muscle mass is not the case in Holy Birman cats. Independently of this aspect, the finding of high creatinine may have a significant effect on clinical decisions, especially when renal failure is suspected, as the current staging system proposed by the International Renal Interest Society classify any cat with serum creatinine concentrations >140 µmol/l, 33 which are actually normal in this breeds, as affected by renal azotaemia (stage II–IV).
High concentrations of glucose have been previously reported for this breed and attributed to stress or a predisposition to diabetes mellitus. 13 In our study, glucose concentration for this breed had a wide distribution of data, rather than a higher RI. Similarly, the high upper reference limit for ALT was due to the skewed distribution of data, with results for most of the cats falling within the RI. Similarly to Abyssinian cats, Holy Birman cats had high serum concentrations of α2-globulin, owing, possibly, to increased concentrations of APP, which migrate in the α2 fraction, 34 or reflecting issues with the textbook reference interval, as previously discussed for electrophoretograms. 22 Clinical signs of inflammation were not detected in these cats. A severe increase of APPs is a hallmark of feline infectious peritonitis (FIP), and Holy Birman cats are highly susceptible to FIP. 35 However, APPs increase also in other conditions 36 and the RI of γ-globulins, which would be expected to increase in cats with FIP, was lower than the published RI. It is, however, worth noting that all four breeds had γ-globulins less than the textbook RI, suggesting issues with this standard. Thus, a relationship between the high α2-globulin concentration and the high prevalence of FIP in this breed cannot be established by the present study.
In Norwegian Forest cats, the RIs for β2- and γ-globulin were lower, perhaps suggesting decreased immune responsiveness. 37 However, all four breeds, again, had decreased RIs for these analytes, so it is more likely to reflect issues with the textbook RI than inadequate immune response, especially as there are no reports of increased infectious disease susceptibility in these cats. Norwegian Forest cats had increased ALP activity, and calcium and phosphate concentrations. Kittens were excluded from this study; therefore, this finding cannot be owing to the bone remodelling typical of young animals. 38 However, it may suggest an active bone metabolism in adult Norwegian Forest cats, possibly owing to their larger body size compared with other breeds. This hypothesis, not supported by data from the literature, merits further investigation.
Low concentrations of β2- and γ-globulins could also suggest decreased immune responsiveness in Siberian cats; however, this has not been noted in the literature and, again, it more likely reflects issues with the textbook RI used for comparison. Wide RIs for creatinine, urea (similar in all four breeds), cholesterol (similarly in the other breeds) and glucose concentrations were found in this breed. It is interesting to note the similarities of the electrophoresis results in Norwegian Forest and Siberian cats. While this could suggest a genetic relationship between these breeds, this is not supported by phylogenetic analyses of feline DNA. 39
Conclusions
This study demonstrated many clinicopathological peculiarities in Abyssinian, Holy Birman, Norwegian Forest and Siberian cats compared with RIs reported in veterinary textbooks. Within each breed, new RIs were established for many analytes, but, in most cases, the new RIs overlapped with the published RIs and the use of the latter does not affect the clinical interpretation of laboratory results. However, some breed peculiarities warranting a specific RI were found. These included RBC microcytosis and a high serum concentration of α2-globulin in Abyssinian cats; high concentrations of creatinine, α2-globulin and, to a lesser extent, glucose in Holy Birman cats; high concentrations of calcium and phosphate, and increased ALP activity in Norwegian Forest cats; and low concentrations of β2- and γ-globulin in Norwegian Forest and Siberian cats, although the decrease of these electrophoretic fractions should be carefully considered as it may have been affected by the inadequacy of the RIs published in textbooks and used for comparison. Further studies are needed to elucidate the pathophysiology of these peculiarities or the possible association with hereditary diseases typical of each breed. However, this study highlights that breed-specific RIs should be used for some analytes to avoid misinterpretation of laboratory results in these breeds.
Supplemental Material
Details of the new reference intervals established in Abyssinian cats reported according to the NCCLS and ASVCP guidelines
Details of the new reference intervals established in Holly Birman cats reported according to the NCCLS and ASVCP guidelines
Details of the new reference intervals established in Norwegian Forest cats reported according to the NCCLS and ASVCP guidelines
Details of the new reference intervals established in Norwegian Forest cats reported according to the NCCLS and ASVCP guidelines
Acknowledgments
We are grateful to the breeders of the cats included in this study and, in particular, to the Italian association of breeders of Holy Birman cats (Agabi) and to the Italian Club of Abyssinian cats (Cigas).
Footnotes
Funding: This study was funded, in part, by the Winn Feline Foundation and by the European Social Fund (Fondo Sociale Europeo, Regione Lombardia), through the ‘Dote Ricerca’ grant.
The authors do not have any potential conflicts of interest to declare.
Supplementary material: Statistical details of the new reference intervals are reported in Supplementary Tables S1–S4.
Accepted: 5 July 2013
References
- 1. Lefebvre HP. Greyhound-specific reference intervals: a good start to a long race. Vet Clin Pathol 2011; 40: 405–406 [DOI] [PubMed] [Google Scholar]
- 2. Geffre A, Friedrichs K, Harr K, et al. Reference values: a review. Vet Clin Pathol 2009; 38: 288–298. [DOI] [PubMed] [Google Scholar]
- 3. National Committee for Clinical Laboratory Standards. Defining, establishing and verifying reference intervals in the clinical laboratory. Approved guideline. 3rd ed. Clinical and Laboratory Standards Institute (CLSI) document C28-A3c. Wayne, PA: CLSI, 2010. [Google Scholar]
- 4. Friedrichs KR, Harr KE, Freeman KP, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 2012; 41: 441–453. [DOI] [PubMed] [Google Scholar]
- 5. Walton RM. Subject-based reference values: biological variation, individuality, and reference change values. Vet Clin Pathol 2012; 41: 175–181. [DOI] [PubMed] [Google Scholar]
- 6. Grahn RA, Grahn JC, Penedo MCT, et al. Erythrocyte pyruvate kinase deficiency mutation identified in multiple breeds of domestic cats. BMC Vet Res. 2012; 8: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pedersen HD, Häggström J, Olsen MJ, et al. Idiopathic asymptomatic thrombocytopenia in Cavalier King Charles spaniels is an autosomal recessive trait. J Vet Intern Med 2002; 16: 169–173. [DOI] [PubMed] [Google Scholar]
- 8. Harvey JW. Microcytic anemias. In: Feldmann BF, Zinkl JG, Jain NC. (eds). Schalm’s veterinary hematology, 5th ed. Philadelphia, PA: Lippincott Williams and Wilkins, 2000, pp 204. [Google Scholar]
- 9. Tisdall PL, Hunt GB, Tsoukalas G, et al. Post-prandial serum bile acid concentrations and ammonia tolerance in Maltese dogs with and without hepatic vascular anomalies. Aust Vet J 1995; 72: 121–126. [DOI] [PubMed] [Google Scholar]
- 10. Zaldívar-López S, Marin LM, Iazbik MC, et al. Clinical pathology of greyhounds and other sighthounds. Vet Clin Pathol 2011; 40: 414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Brien SJ, Johnson W, Driscoll C, et al. State of cat genomics. Trend Genet 2008; 24: 268–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyons LA. Feline genetics: clinical applications and genetic testing. Top Companion Anim Pract 2010; 25: 203–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reynolds BS, Concordet D, Germain CA, et al. Breed dependency of reference intervals for plasma biochemical values in cats. J Vet Intern Med 2010; 24: 809–818. [DOI] [PubMed] [Google Scholar]
- 14. Lilliehöök I, Tvedten H. Validation of the Sysmex XT-2000iV hematology system for dogs cats and horses. I. Erythrocytes, platelets, and total leukocyte counts. Vet Clin Pathol 2009; 38: 163–174. [DOI] [PubMed] [Google Scholar]
- 15. Norman EJ, Barron RCJ, Nash AS, et al. Prevalence of low automated platelet counts in cats: comparison with prevalence of thrombocytopenia based on blood smear estimation. Vet Clin Pathol 2001; 30: 137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Granat F, Geffré A, Braun JP, et al. Comparison of platelet clumping and complete blood count results with Sysmex XT-2000iV in feline blood sampled on EDTA or EDTA plus CTAD (citrate, theophylline, adenosine and dipyridamole). J Feline Med Surg 2011; 13: 953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaneko JJ, Harvey JW, Bruss ML. Blood analyte reference values in small and some laboratory animals. In: Kaneko JJ, Harvey JW, Bruss ML. (eds). Clinical biochemistry of domestic animals. 5th ed. San Diego, CA: Academic Press, 1997, pp 885–905. [Google Scholar]
- 18. Rizzi TE, Clinkenbeard KD, Meinkoth JH. Normal hematology of the cat. In: Weiss DJ, Wardrop KJ. (eds). Schalm’s veterinary hematology. 6th ed. Ames, IA: Wiley-Blackwell, 2010, pp 811–820. [Google Scholar]
- 19. Geffré A, Concordet D, Braun JP, et al. Reference Value Advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet Clin Pathol 2011; 40: 107–112. [DOI] [PubMed] [Google Scholar]
- 20. Bourgès-Abella N, Geffré A, Concordet D, et al. Canine reference intervals for the Sysmex XT-2000iV hematology analyzer. Vet Clin Pathol 2011; 40: 303–315. [DOI] [PubMed] [Google Scholar]
- 21. Tvedten H, Moritz A. Reticulocyte and Heinz body staining and enumeration. In: Weiss DJ, Wardrop KJ. (eds). Schalm’s veterinary hematology, 6th ed. Ames, IA: Wiley-Blackwell, 2010, pp 1067–1074. [Google Scholar]
- 22. Giordano A, Paltrinieri S. Interpretation of capillary zone electrophoresis compared with cellulose acetate and agarose gel electrophoresis: reference intervals and diagnostic efficiency in dogs and cats. Vet Clin Pathol 2010; 39: 464–473. [DOI] [PubMed] [Google Scholar]
- 23. Tvedten H, Johansson P. Feline platelet counting with prostaglandin E1 on the Sysmex XT-2000iV. Vet Clin Pathol 2010; 39: 190–192. [DOI] [PubMed] [Google Scholar]
- 24. Granat F, Geffré A, Bourgès-Abella N, et al. Changes in haematology measurements with the Sysmex XT-2000iV during storage of feline blood sampled in EDTA or EDTA plus CTAD. J Feline Med Surg 2013; 15: 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tasker S, Cripps PJ, Mackin AJ. Estimation of platelet counts on feline blood smears. Vet Clin Pathol 1999; 28: 42–45. [DOI] [PubMed] [Google Scholar]
- 26. Fujise H, Hishiyama N, Ochiai H. Heredity of red blood cells with high K and low glutathione (HK/LG) and high K and high glutathione (HK/ HG) in a family of Japanese Shiba dogs. Exp Anim 1997; 46: 41–46. [DOI] [PubMed] [Google Scholar]
- 27. Sheehan RG, Frenkel EP. Influence of hemoglobin phenotype on the mean erythrocyte volume. Acta Haematol 1983; 69: 260–265. [DOI] [PubMed] [Google Scholar]
- 28. Kohn B, Henthorn PS, Rajpurohit Y, et al. Feline adult beta-globin polymorphism reflected in restriction fragment length patterns. J Hered 1999; 90: 177–181. [DOI] [PubMed] [Google Scholar]
- 29. DiBartola SP, Tarr MJ, Benson MD. Tissue distribution of amyloid deposits in Abyssinian cats with familial amyloidosis. J Comp Pathol 1986; 4: 387–398. [DOI] [PubMed] [Google Scholar]
- 30. DiBartola SP, Reiter JA, Kociba GJ, et al. Serum amyloid A protein concentration measured by radial immunodiffusion an Abyssinian and non-Abyssinian cats. Am J Vet Res 1989; 50: 1414–1417. [PubMed] [Google Scholar]
- 31. Eklund KK, Niemi K, Kovanen PT. Immune functions of serum amyloid A. Crit Rev Immunol 2012; 32: 335–348. [DOI] [PubMed] [Google Scholar]
- 32. Gunn-Moore DA, Dodkin SJ, Sparkes AH. An unexpectedly high prevalence of azotaemia in Birman cats. J Feline Med Surg 2002; 4: 165–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elliott J, Watson ADJ. Chronic kidney disease: staging and management. In: Bonagura J, Twedt D. (eds). Kirk’s current veterinary therapy. 14th ed. St Louis, MO: Saunders, 2008, pp 883–892 [Google Scholar]
- 34. Eckersall DP. Proteins, proteomics, and the dysproteinemias. In: Kaneko JJ, Harvey JW, Bruss ML. (eds). Clinical biochemistry of domestic animals. 6th ed. San Diego, CA, Elsevier, 2008, pp 117–156. [Google Scholar]
- 35. Pesteanu-Somogyi LD, Radzai C, Pressler BM. Prevalence of feline infectious peritonitis in specific cat breeds. J Feline Med Surg 2006; 8: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paltrinieri S. The feline acute phase reaction. Vet J 2008; 177: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zemble RM, Takach PA, Levinson AI. The relationship between hypogammaglobulinemia, monoclonal gammopathy of undetermined significance and humoral immunodeficiency: a case series. J Clin Immunol 2011; 31: 737–743 [DOI] [PubMed] [Google Scholar]
- 38. Fernandez NJ, Kidney BA. Alkaline phosphatase: beyond the liver. Vet Clin Pathol 2007; 36: 223–233. [DOI] [PubMed] [Google Scholar]
- 39. Lipinski MJ, Froenicke L, Baysac KC. The ascent of cat breeds: genetic evaluations of breeds and worldwide random-bred populations. Genomics 2008; 91: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of the new reference intervals established in Abyssinian cats reported according to the NCCLS and ASVCP guidelines
Details of the new reference intervals established in Holly Birman cats reported according to the NCCLS and ASVCP guidelines
Details of the new reference intervals established in Norwegian Forest cats reported according to the NCCLS and ASVCP guidelines
Details of the new reference intervals established in Norwegian Forest cats reported according to the NCCLS and ASVCP guidelines