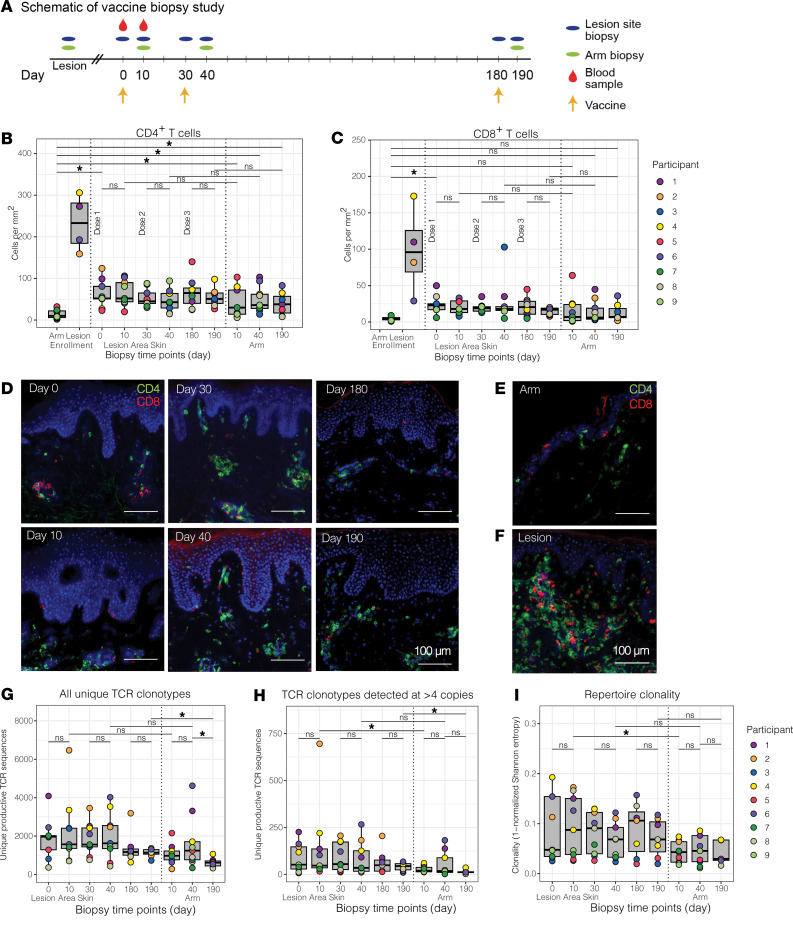
Figure 1. Number, fold change, and clonality of TCR clonotypes in HSV lesion site and arm biopsies before and after vaccination.
(A) Schematic of vaccine study timeline and procedures. (B) CD4+ and (C) CD8+ T cell densities of biopsies from control skin at the time of enrollment and from the site of a symptomatic lesion (N = 4) are shown in comparison to HSV lesion site and control skin biopsies over the course of a 3-dose vaccine trial in 9 vaccine recipients. Each dot represents the mean of 3 counted sections in a single participant. Median and interquartile range are shown in gray. (D) Representative micrographs (original magnification, ×10) of CD4+ (green) and CD8+ (red) T cell density by immunofluorescence (IF) in HSV lesion site biopsies at specified time points before and after vaccination. CD4+ and CD8+ T cell IF from (E) control skin and (F) lesion site during a symptomatic HSV-2 outbreak. All images are from P4. Scale bars: 100 mm. (G) Total and (H) number of TCRβ clonotypes detected at >4 copies are shown from the HSV lesion site and arm biopsies. (I) Clonality calculated from Shannon entropy of the TCR repertoire from each sample. Each dot represents a single participant. Median and interquartile range are shown in gray. *P < 0.05 by Wilcoxon’s signed-rank test.