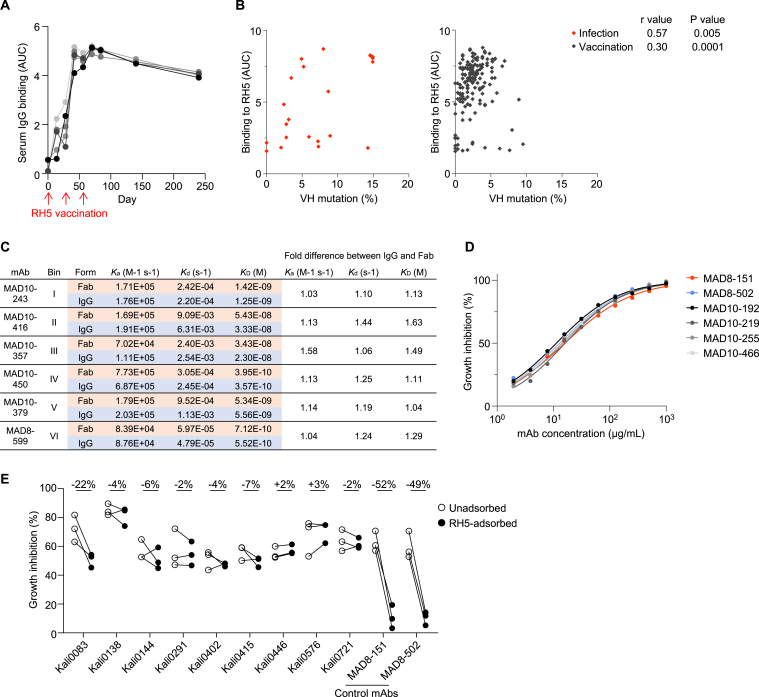
Figure S1.
Infection- and vaccination-induced antibody responses to RH5, related to Figures 1 and 2
(A) Serum IgG reactivity to RH5 after three doses of the RH5/AS01 vaccine in five malaria-naive individuals.
(B) Association between RH5 binding and VH mutations of RH5-specific mAbs from natural infection (red) and vaccination (black). p and r values were calculated based on Spearman correlation.
(C) Binding kinetics of RH5-specific IgG (targeting bins I–VI) and corresponding Fabs to RH5. Equimolar binding arms of each form (4.2 nM Fab and 2.1 nM IgG) were compared. Ka, association rate constant; Kd, dissociation rate constant; KD, equilibrium dissociation constant.
(D) Growth inhibition titration curves of the six most potent RH5-specific mAbs. Data are shown from a representative experiment out of n = 2–3 experiments. MAD8–151 and MAD8–502 were isolated from infected donors while MAD10–192, MAD10–219, MAD10–255, and MAD10–466 were isolated from vaccinated donors.
(E) Growth inhibition mediated by polyclonal IgG from naturally infected donors in an antigen-reversal assay, where samples are tested for activity with and without adsorption of RH5-specific antibodies with soluble antigen. Each pair of points represents an independent experiment. MAD8–151 and MAD8–502 are control RH5-specific mAbs. Kali0083 is the source donor of MAD8–502 and Kali0446 is the source donor of MAD8–151. The percentages at the top of the figure refer to the mean difference between the RH5-adsorbed and -unadsorbed inhibition values.