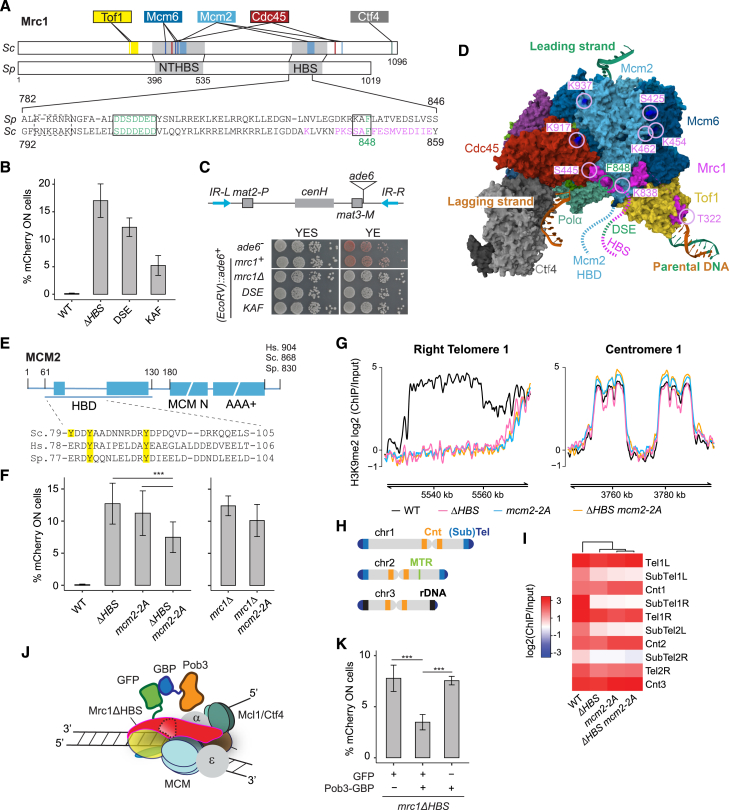
Figure 3.
Coordinated function of Mrc1 and Mcm2-HBD in heterochromatin maintenance
(A) Aligned S. cerevisiae and S. pombe Mrc1 proteins showing contacts and cross-links to replisome components in S. cerevisiae2,4 and highly conserved residues in Mrc1 HBS (bottom). Boxed residues were mutated in this study. Magenta residues are identified in the cryo-EM structure.4
(B) Cells expressing mCherry (n = 6). Data are represented as mean ± SD.
(C) Expression of ade6+ reporter in mrc1 mutants. Red color on YE medium indicates repression.
(D) Mrc1 contacts with replisome components shown in magenta on PDB 8B9A4 with the expected positions of the Mcm2 HBD and Mrc1 HBS and DSE indicated. Cross-linked residues (from Baretić et al. [2020]2) are indicated by magenta circles and labeled by their position in S. cerevisiae Mrc1. The conserved F848 in KAF is in green.
(E) Conserved tyrosine residues in the Mcm2 HBD mutated to alanine in mcm2-2A.
(F) Cells expressing mCherry (n = 18 [left] [ANOVA, F = 73.9, p = 2.2 × 10−16]; n = 6 [right]). Data are represented as mean ± SD.
(G) H3K9me2 in subtelomeric region Tel1R in mrc1ΔHBS and mcm2-2A mutants. Centromere 1 is shown for comparison.
(H) Major heterochromatic regions of S. pombe.
(I) Heatmap depicting H3K9me2 at heterochromatic regions.
(J and K) Tethering Pob3 to Mrc1ΔHBS through a GFP-GBP interaction (J) restores silencing of mCherry reporter (K). (n = 6) [ANOVA, F = 45.9, p = 7.1 × 10−7]. Data are represented as mean ± SD.
See also Figures S2 and S3.