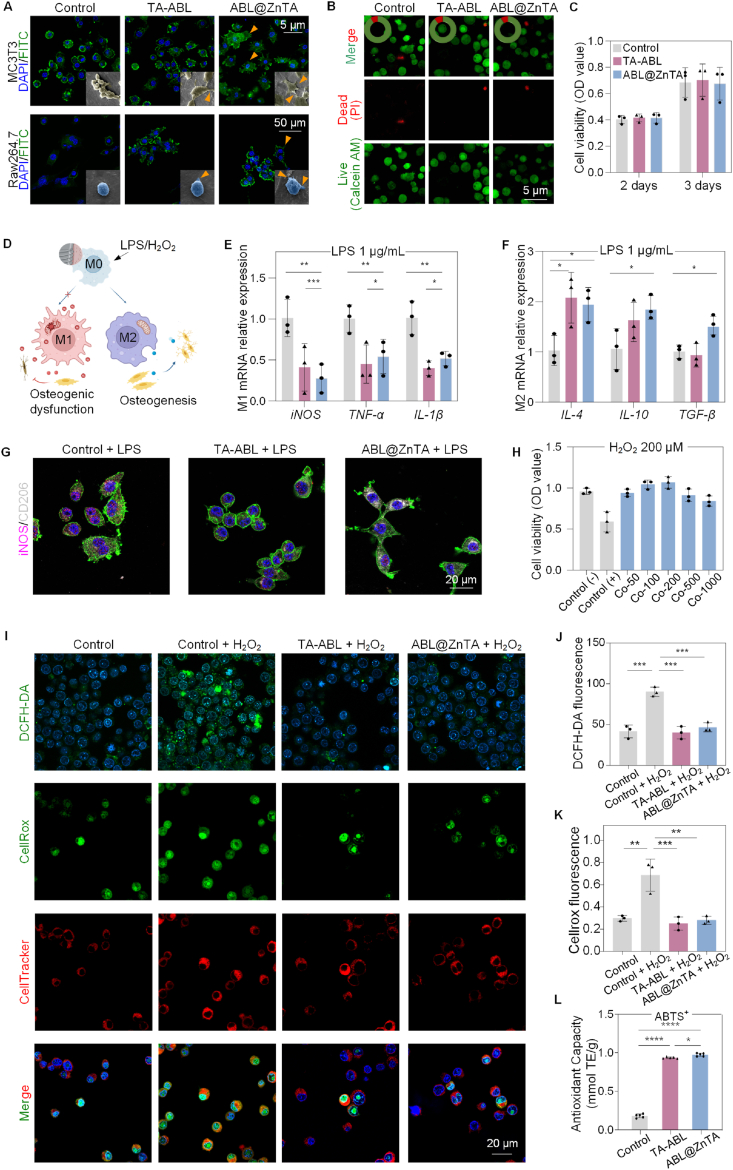
Fig. 4.
Biocompatibility, anti-inflammatory, and immunomodulatory activity of the ABL@ZnTA nanointerface. (A) CLSM and SEM observation of MC3T3 and RAW264.7 cells morphology seeded on with various coatings for 2 days. Nucleus and cellular actin filaments of cells are labeled with the DAPI (blue) and FITC (green). The arrowheads represent the cellular pseudopodia. For SEM, the MC3T3 cells are pseudo-colored to yellow and RAW264.7 to blue for visual observation. (B) Calcein-AM/PI double staining of RAW264.7 cells for 2 days (Calcein AM-positive cells, green; PI-positive cells, red); semi-quantitative analysis of the proportion of live and dead cells in the top left of images. (C) CCK-8 results of RAW264.7 cells for 2 and 3 days. (D) Schematic diagram of macrophage polarization under LPS/H2O2 stimulus and subsequent effect on osteogenesis. (E and F) Relative mRNA expression of M1 and M2-related genes in macrophage under stimulation of 1 μg/mL LPS detected for 2 days by qRT-PCR analysis, n = 3. (G) Immunofluorescence of iNOS and CD206 of macrophage under 1 μg/mL LPS stimulation for 2 days. (H) CCK-8 result of macrophages on the ABL@ZnTA coating of different concentrations stimulated with H2O2 (200 μM) for 12 h, n = 3. (I–K) Representative fluorescence images and quantification of DCFH-DA, CellRox, and CellTracker staining for intracellular ROS stimulated with H2O2 (200 μM) for 2 h, n = 3. (L) Total antioxidant capacity with ABTS method result of extracellular ROS for 1 day, n = 5. Data are presented as mean ± SD. Statistical significance was calculated via one-way ANOVA with Tukey posthoc test (*, P < 0.05; **, P < 0.01; and ***, P < 0.001).