Abstract
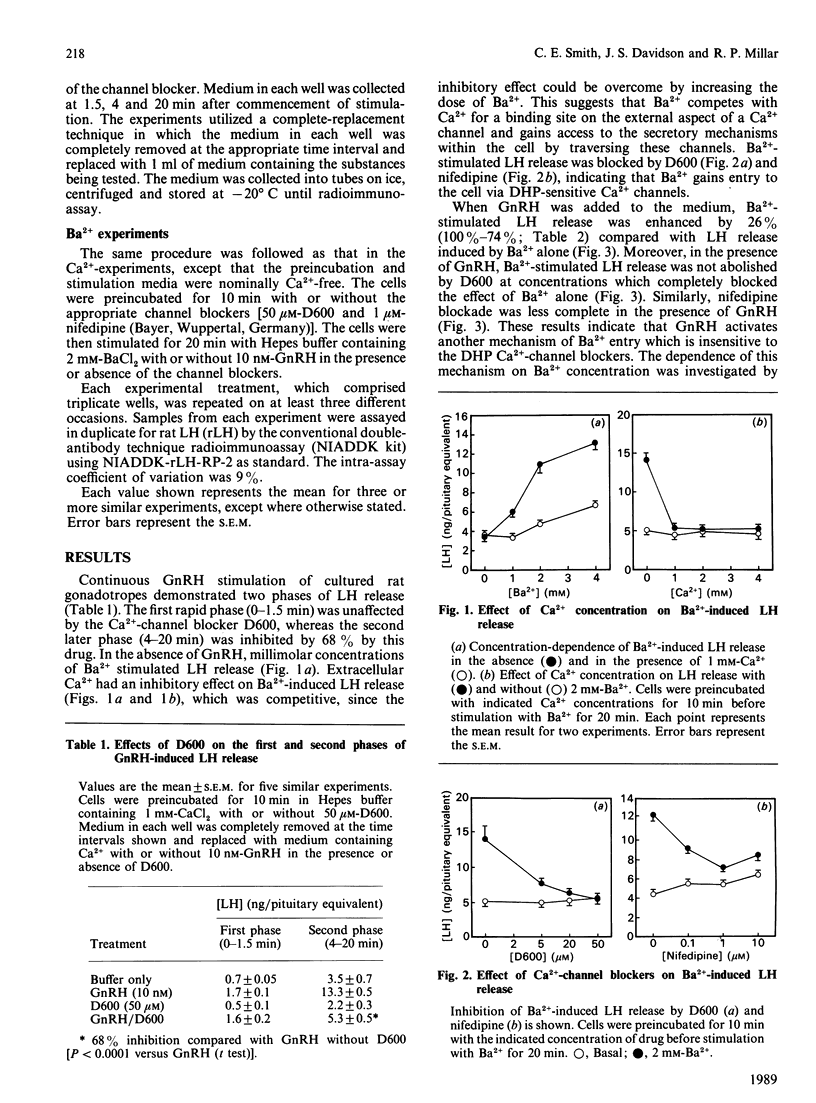
Kinetic studies on gonadotropin-releasing-hormone (gonadoliberin, GnRH)-stimulated luteinizing-hormone (lutropin, LH) release in the cultured rat gonadotrope demonstrated a biphasic pattern of LH release. The first rapid phase of release was unaffected by the voltage-gated Ca2+-channel blockers methoxyverapamil (D600) and nifedipine [a dihydropyridine (DHP)], whereas the later second phase was partially inhibited by both drugs. These results suggested that the initial phase of LH release is independent of Ca2+ entry through dihydropyridine (DHP)-sensitive Ca2+ channels and might depend on entry of extracellular Ca2+ by another mechanism. These mechanisms were further studied by utilizing Ba2+ as a Ca2+ substitute. Ba2+, which freely permeates DHP-sensitive Ca2+ channels in the absence of GnRH, induced LH release which was sensitive to blockade by D600 and nifedipine. However, in the presence of the channel blockers, Ba2+-induced LH release could be elicited when GnRH was added to the system. This indicates that GnRH stimulates LH release by initially activating a DHP-insensitive Ca2+-entry mechanism and then a DHP-sensitive mechanism. The DHP-sensitive mechanism freely allows Ba2+ entry in the absence of GnRH-receptor occupancy, whereas the DHP-insensitive mechanism requires GnRH-receptor activation for Ba2+ entry.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W. V., Conn P. M. Gonadotropin-releasing hormone stimulates mass changes in phosphoinositides and diacylglycerol accumulation in purified gonadotrope cell cultures. Endocrinology. 1986 Mar;118(3):1148–1158. doi: 10.1210/endo-118-3-1148. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Borges J. L., Scott D., Kaiser D. L., Evans W. S., Thorner M. O. Ca+2 dependence of gonadotropin-releasing hormone-stimulated luteinizing hormone secretion: in vitro studies using continuously perifused dispersed rat anterior pituitary cells. Endocrinology. 1983 Aug;113(2):557–562. doi: 10.1210/endo-113-2-557. [DOI] [PubMed] [Google Scholar]
- Bourne G. A., Baldwin D. M. Extracellular Ca++-independent and -dependent components of the biphasic release of LH in response to luteinizing hormone-releasing hormone in vitro. Endocrinology. 1980 Sep;107(3):780–788. doi: 10.1210/endo-107-3-780. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Seagar M. J., Takahashi M. Molecular properties of dihydropyridine-sensitive calcium channels in skeletal muscle. J Biol Chem. 1988 Mar 15;263(8):3535–3538. [PubMed] [Google Scholar]
- Childs G. V., Marchetti C., Brown A. M. Involvement of sodium channels and two types of calcium channels in the regulation of adrenocorticotropin release. Endocrinology. 1987 May;120(5):2059–2069. doi: 10.1210/endo-120-5-2059. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. STIMULANT ACTION OF BARIUM ON THE ADRENAL MEDULLA. Nature. 1964 Jul 18;203:305–307. doi: 10.1038/203305a0. [DOI] [PubMed] [Google Scholar]
- Davidson J. S., Wakefield I. K., King J. A., Mulligan G. P., Millar R. P. Dual pathways of calcium entry in spike and plateau phases of luteinizing hormone release from chicken pituitary cells: sequential activation of receptor-operated and voltage-sensitive calcium channels by gonadotropin-releasing hormone. Mol Endocrinol. 1988 Apr;2(4):382–390. doi: 10.1210/mend-2-4-382. [DOI] [PubMed] [Google Scholar]
- Davidson J. S., Wakefield I., King J. A., Millar R. P. Barium-induced LH release from chicken pituitary cells: synergism with phorbol ester. J Endocrinol. 1987 Jul;114(1):11–16. doi: 10.1677/joe.0.1140011. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S., Tomiko S. A. Secretagogue effect of barium on output of melanocyte-stimulating hormone from pars intermedia of the mouse pituitary. J Physiol. 1983 May;338:243–257. doi: 10.1113/jphysiol.1983.sp014671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:431–446. doi: 10.1113/jphysiol.1985.sp015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The role of the alkaline earth ions in anaphylactic histamine secretion. J Physiol. 1972 Aug;224(3):753–769. doi: 10.1113/jphysiol.1972.sp009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. R., Dineen D. X., Petrak R. Mechanism of action of barium ion on rat aortic smooth muscle. Am J Physiol. 1984 Mar;246(3 Pt 1):C235–C241. doi: 10.1152/ajpcell.1984.246.3.C235. [DOI] [PubMed] [Google Scholar]
- Hardcastle J., Hardcastle P. T., Noble J. M. The secretory action of barium chloride in rat colon. J Physiol. 1985 Apr;361:19–33. doi: 10.1113/jphysiol.1985.sp015630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C. R. Short term kinetics of luteinizing hormone secretion studied in dissociated pituitary cells attached to manipulable substrates. J Cell Biol. 1977 Jun;73(3):685–695. doi: 10.1083/jcb.73.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreye V. A., Hofmann F., Mühleisen M. Barium can replace calcium in calmodulin-dependent contractions of skinned renal arteries of the rabbit. Pflugers Arch. 1986 Mar;406(3):308–311. doi: 10.1007/BF00640919. [DOI] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Limor R., Ayalon D., Capponi A. M., Childs G. V., Naor Z. Cytosolic free calcium levels in cultured pituitary cells separated by centrifugal elutriation: effect of gonadotropin-releasing hormone. Endocrinology. 1987 Feb;120(2):497–503. doi: 10.1210/endo-120-2-497. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Properties of two types of calcium channels in clonal pituitary cells. J Gen Physiol. 1986 Jan;87(1):161–182. doi: 10.1085/jgp.87.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Naor Z., Catt K. J. Mechanism of action of gonadotropin-releasing hormone. Involvement of phospholipid turnover in luteinizing hormone release. J Biol Chem. 1981 Mar 10;256(5):2226–2229. [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Smith C. E., Wakefield I., King J. A., Naor Z., Millar R. P., Davidson J. S. The initial phase of GnRH-stimulated LH release from pituitary cells is independent of calcium entry through voltage-gated channels. FEBS Lett. 1987 Dec 10;225(1-2):247–250. doi: 10.1016/0014-5793(87)81167-5. [DOI] [PubMed] [Google Scholar]
- Tasaka K., Stojilkovic S. S., Izumi S., Catt K. J. Biphasic activation of cytosolic free calcium and LH responses by gonadotropin-releasing hormone. Biochem Biophys Res Commun. 1988 Jul 15;154(1):398–403. doi: 10.1016/0006-291x(88)90699-7. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., Prod'hom B., Baggiolini M., Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. 1986 Nov 27-Dec 3Nature. 324(6095):369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]


