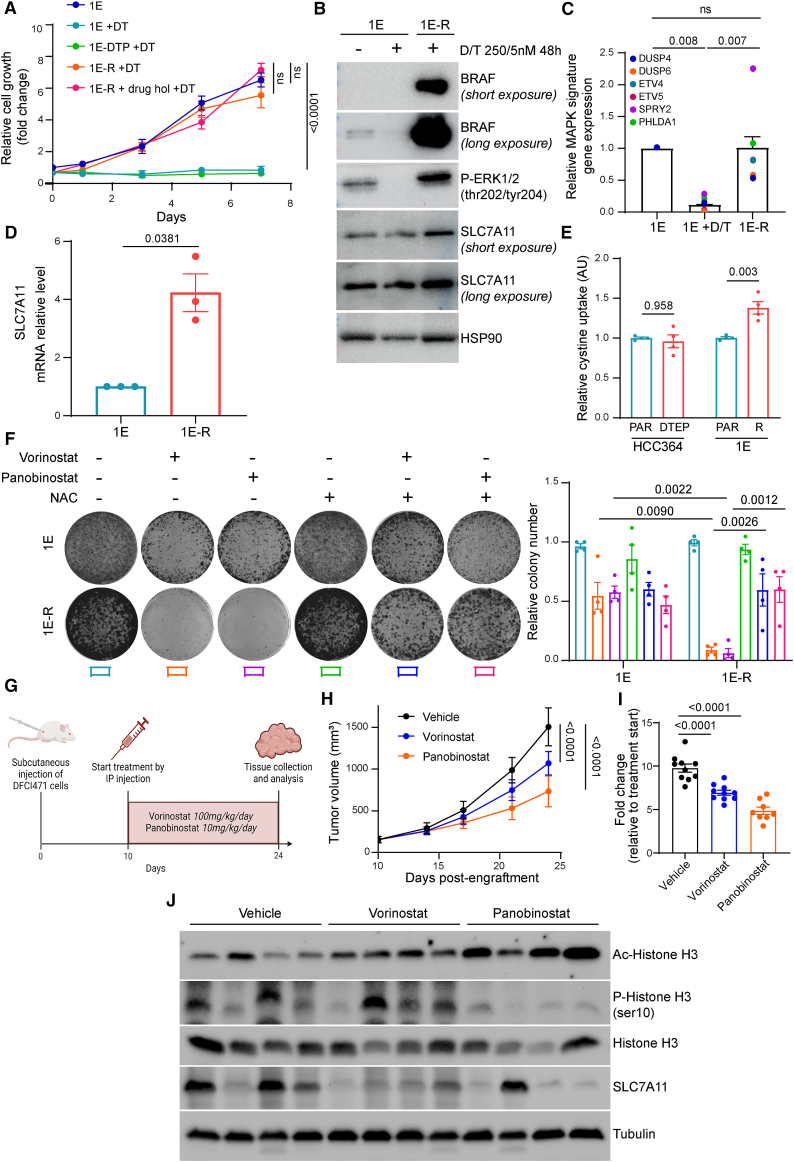
Figure 6.
MAPK pathway reactivation through BRAF amplification is associated with ROS vulnerabilities and in vivo targeting of NRAS-mediated D/T resistance in BRAFV600E LUAD cells with HDAC inhibitors
(A) DTPs and 1E-R cells were generated by maintaining BRAFV600E-mutant 1E-PDX cells on increasing concentrations of D/T treatment for 3 and 30 weeks, respectively. Growth curves of parental, DTP, and -R 1E cells in the presence of 250/5 nM D/T. 1E-R cells underwent a drug holiday for 3 weeks and were rechallenged with D/T to confirm the genetic adaptation of these cells to the treatment (1E-R + drug hol + D/T). Data were analyzed using two-way ANOVA followed by Tukey’s multiple comparisons post-test and are shown as the mean values ± SEM. n = 3 biological replicates.
(B) Immunoblot of BRAF, phospho-ERK (p-ERK), and SLC7A11 in 1E and 1E-R cells cultured in the presence of 250/5 nM D/T for 48 h. HSP90 was used as a loading control. n = 2 biological replicates.
(C) Quantitative RT-PCR analysis of relative MAPK pathway target genes (DUSP4, DUSP6, ETV4, ETV5, SPRY2, and PHLDA1) mRNA levels in 1E and 1E-R cells cultured in the presence of 250/5 nM D/T for 48 h. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparisons post-test and are shown as the mean values ±SEM. n = 3 biological replicates.
(D) Quantitative RT-PCR analysis of SLC7A11 mRNA level in 1E and 1E-R cells. Data were analyzed using unpaired t test with Welch’s correction and are shown as the mean values ± SEM. n = 3 biological replicates.
(E) Intracellular cystine uptake in HCC364 (parental and DTEP) and 1E (parental and -R) cells. Data were analyzed using two-way ANOVA followed by Tukey’s multiple comparisons post-test and are shown as the mean values ± SEM. n = 4 biological replicates.
(F) Clonogenic assay of 1E and 1E-R cells co-treated with 1 μM vorinostat or 10 nM panobinostat and 2.5 mM NAC. Data were analyzed using two-way ANOVA followed by Tukey’s multiple comparisons post-test and are shown as the mean values ± SEM. n = 4 biological replicates.
(G) Schematic outline of the DFCI471 subcutaneous xenograft model. Mice were treated with vorinostat (100 mg/kg/day) or panobinostat (10 mg/kg/day) by intraperitoneal injection 5 days per week. After 14 days, mice were sacrificed and tumors were collected for further analysis.
(H) Follow-up of tumor growth of the indicated conditions. Data are represented as mean tumor volume ±SEM. n = 8 or 10 mice for each group.
(I) Fold change in tumor growth compared to baseline.
(J) Immunoblot of acetyl and phospho-histone H3 (Ac and p-histone H3), histone H3, and SLC7A11 in 4 representative tumors per condition. Tubulin was used as a loading control. n = 4 biological replicates. See also Figure S8.