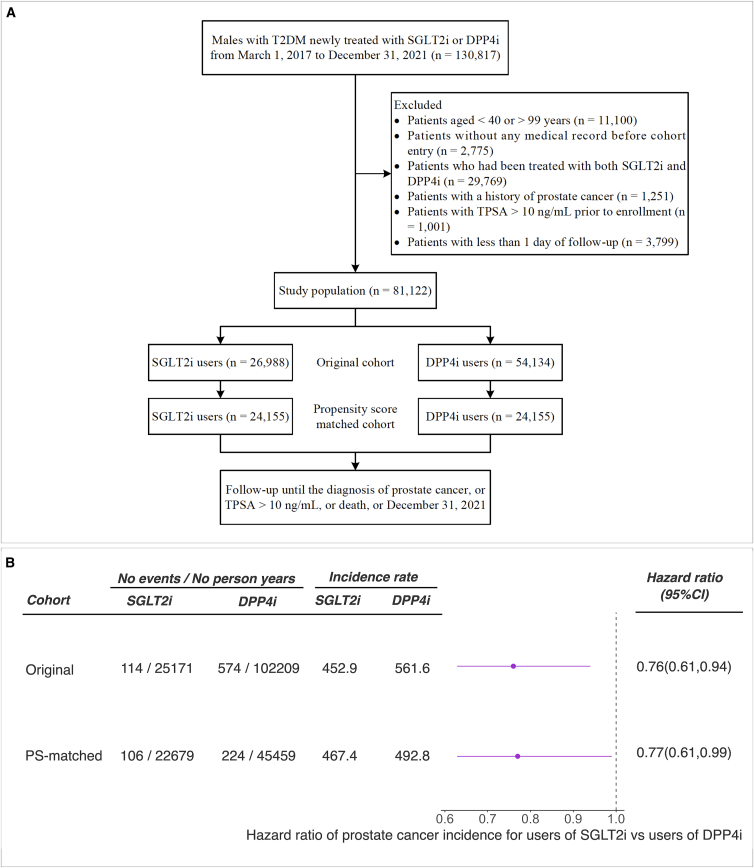
Figure 3.
Flowchart of patient inclusion and association between the use of SGLT2 inhibitors and the risk of incident prostate cancer or being at high risk of prostate cancer
(A) Flowchart of patient inclusion in the study population. SGLT2i, sodium glucose cotransporter 2 inhibitors; DPP4i, dipeptidylpeptidase 4 inhibitors; TPSA, total prostate-specific antigen. A patient could be excluded for more than one reason.
(B) The association between use of SGLT2 inhibitors compared with DPP4 inhibitors and risk of prostate cancer or with total PSA > 10 ng/mL (which indicated high risk of prostate cancer). The covariates used in this analysis include demographic data (age), comorbidities (benign prostatic hyperplasia, hypertension, dyslipidemia, diabetic complications, ischemic heart disease, peripheral vascular disease, heart failure, cerebrovascular disease, chronic lung disease, moderate or severe kidney disease, moderate or severe liver disease, and other cancers), anti-diabetic drugs (metformin, insulin, glucagon-like peptide-1 receptor agonist, sulfonylurea, glinide, α-glucosidase inhibitor, and thiazolidinedione), and other medications (angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, α/β-blockers, diuretic, statin, fibrate, aspirin, other antiplatelet drugs, non-steroidal anti-inflammatory drug, and 5α-reductase inhibitor). The unit of the incidence rate was 100,000 person-years. Harzard ratio is the probability of occurrence of prostate cancer in SGLT2 inhibitor users versus that in DPP4 ihibitor users during the follow-up period.