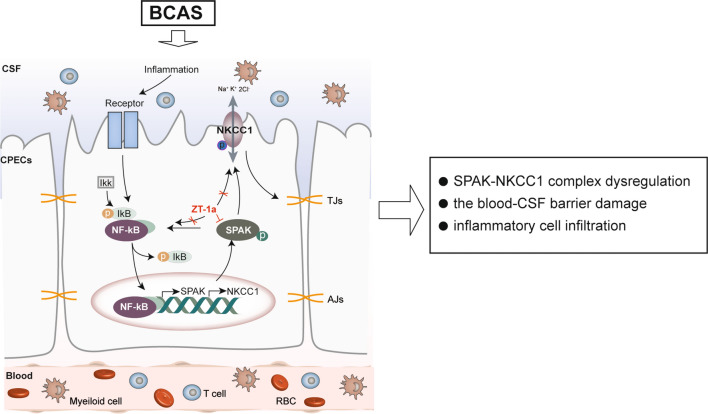
Fig. 10.
Schematic summary of the brain-ChP barrier damage after BCAS. The ChP, with tight junctions (TJ) at the apical membrane of the ChP epithelial cells, prevents inflammation by blocking immune cells from entering the brain. Na+-K+-Cl− cotransporter isoform 1 (NKCC1) and its regulatory serine-threonine kinase, SPAK, located at the apical membrane, are essential in regulating choroid plexus epithelial cell volume and CSF secretion/clearance. BCAS stimulates dynamic changes of TJ proteins and the SPAK-NKCC1 complex, which contributes to altered ChP TJ proteins and infiltration of immune cells. Inflammatory signals activate the IκB kinase (IKK) complex, leading to the phosphorylation and dissociation of IκB proteins (IκBα/β/ε) that normally sequester NF-κB dimers (p65/p50) in the cytoplasm. This process allows NF-κB to move from the cytosol to the nucleus, where it promotes the transcription of target genes like SPAK and NKCC1. Pharmacological inhibition of SPAK with its potent inhibitor ZT-1a provides protective effects via attenuating BCAS-induced structural changes at the ChP and neuroinflammation