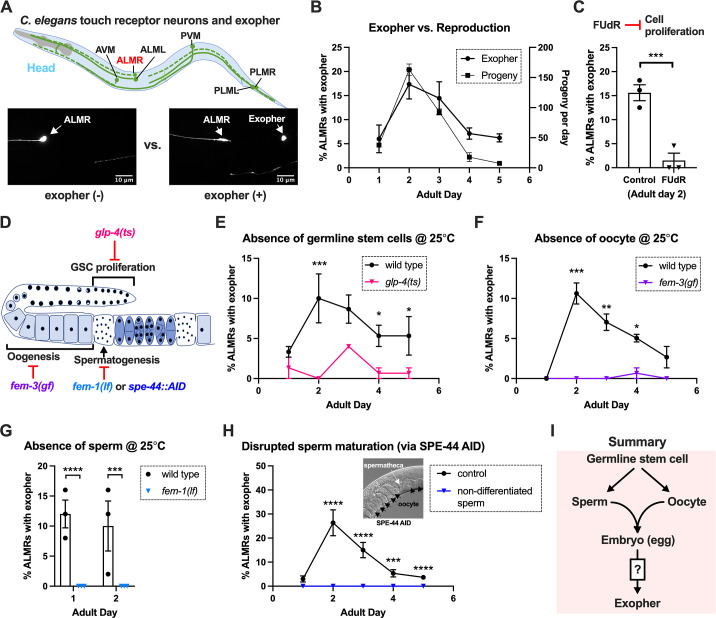
Figure 1. Exophergenesis is dependent on the presence of the germline, ooyctes, and sperm.
(A) Exophers produced by ALMR are readily visualized in living C. elegans. Top, positions of the six C. elegans touch receptor neurons: AVM (Anterior Ventral Microtubule cell), ALMR (Anterior Lateral Microtubule cell, Right), ALML (Anterior Lateral Microtubule cell, Left), PVM (Posterior Ventral Microtubule cell, Right), PLMR (Posterior Lateral Microtubule cell, Right), and PLML (Posterior Lateral Microtubule cell, Left). Bottom panels are representative pictures (n > 100, scale bar = 10 μm) of an ALMR neuron without (lower left) or with (lower right) exopher production from strain ZB4065 bzIs166[Pmec-4::mCherry], which expresses elevated mCherry in the touch receptor neurons. Over-expression of mCherry in bzIs166 is associated with enlargement of lysosomes and formation of large mCherry foci that often correspond to LAMP::GFP-positive structures; ultrastructure studies reveal considerable organelle morphological change not seen in low reporter-expression neurons; polyQ74, polyQ128, Aβ1-42 over-expression all increase exophers (Melentijevic et al., 2017; Arnold et al., 2023). Most genetic compromise of different proteostasis branches--heat shock chaperones, proteasome, and autophagy--enhance exophergenesis, supporting exophergenesis as a response to proteostress. (B) Both exopher production and reproduction typically peak around Ad2 in the Pmec-4::mCherry strain. Left axis: the frequency of exopher events in the adult hermaphrodite C. elegans strain ZB4065 at 20 °C; Mean ± SEM of nine independent trials (~50 animals in each trial). Right axis: daily progeny count (Mean ± SEM) from 10 wild-type N2 hermaphrodites. (C) 5-fluoro-2'-deoxyuridine (FUdR), which inhibits progeny production, suppresses early adult exopher production. Data are the percentage of ALMR exopher events among >50 Ad2 hermaphrodites in each trial (total of 3 independent trials) for strain ZB4065 at 20 °C in absence (control) or presence of 51 μM FUDR. ***p<0.001 in Cochran–Mantel–Haenszel test. (D) Illustration of the roles of germline development genes tested for impact on exophers. The C. elegans reproductive system comprises a bilobed gonad in which germ cells (light blue, dark nuclei) develop into mature oocytes, which are fertilized in the spermatheca (sperm indicated as dark dots) and held in the uterus until about the 30-cell gastrulation stage, at which point eggs (dark blue) are laid. Indicated are the steps impaired by germline developmental mutations we tested. (E) Germline stem cells are required for efficient exopher production. Data show the percentage of ALMR exopher events (Mean ± SEM) among 50 adult hermaphrodite C. elegans that express the wild-type GLP-4 protein or the GLP-4(ts) protein encoded by glp-4(bn2) at 25 °C. All animals express Pmec-4mCherry, and the glp-4(ts) mutants lack a germline when reared at the restrictive temperature (25 °C). Eggs collected from both wild-type and the glp-4(ts) mutants were grown at 15 °C for 24 hr before being shifted to 25 °C (at L1 stage) to enable development under restrictive conditions; three independent trials of 50 animals represented by each dot; ***p<0.001 or *p<0.05 in Cochran–Mantel–Haenszel test. Note that in wild-type (WT), temperature shift normally induces a modest elevation in exopher numbers (typically a few % increase, supplemental data in Cooper et al., 2021) and is thus not itself a factor in exopher production levels. (F) Oogenesis is required for efficient exopher production. Spermatogenesis occurs but oogenesis is blocked when fem-3(gf) mutant hermaphrodites are cultured at 25°C. Data show the percentage of ALMR exopher events (Mean ± SEM) in hermaphrodite C. elegans that express the wild-type FEM-3 protein or the temperature-dependent gain-of-function (gf) FEM-3 protein (25 °C; three independent trials, n=50/trial), ***p<0.001; **p<0.01; or *p<0.05 in the Cochran–Mantel–Haenszel test. (G) Spermatogenesis is required for efficient exopher production. There is no spermatogenesis in fem-1(lf) at the restrictive temperature of 25 °C, while oogenesis is normal in the hermaphrodite. Shown is the percentage of ALMR exopher events (Mean ± SEM) in adult hermaphrodites that express the wild-type FEM-1 protein or the temperature-dependent loss of function (lf) FEM-1 protein (25 °C; three independent trials, 50 animals/trial). ****p<0.0001 or ***p<0.001 in Cochran–Mantel–Haenszel test. (H) Spermatogenesis is required for efficient exopher production. Data show the percentage of ALMR exopher events (Mean ± SEM) in hermaphrodite C. elegans that express the SPE-44::degron fusion. SPE-44 is a critical transcription factor for spermatogenesis (Kulkarni et al., 2012), and is tagged with a degron sequence that enables targeted degradation in the presence of auxin in line ZB4749. In the auxin-inducible degron (AID) system, auxin is added to the plates in 0.25% ethanol, so ‘control’ is treated with 0.25% ethanol and ‘no sperm’ is treated with 1 mM auxin applied to plates in 0.25% ethanol from egg to adult day 1; 4–6 independent trials of 50 animals per trial. ****p<0.0001 or ***p<0.001 in Cochran–Mantel–Haenszel test. Note that under no sperm or non-functional sperm production, oocytes still transit through the spermatheca and enter the uterus (as shown by the DIC picture); unfertilized oocytes can be laid if the egg-laying apparatus is intact. (I) Summary: Genetic interventions that block major steps of germ cell development strongly block ALMR exophergenesis in the adult hermaphrodite. The dual requirement for sperm and oocytes suggests that fertilization and embryogenesis are required events for inducing ALMR exophergenesis.