Abstract
Background
Primary tumor (PT) sidedness is an established prognostic marker in metastatic colorectal cancer (mCRC) and has a predictive impact on the efficacy of anti-epidermal growth factor receptor (anti-EGFR) antibody [monoclonal antibody (mAb)] in patients with RAS wild-type mCRC. This investigation focuses on patients with BRAFV600E-mutated (BRAFmt) mCRC and examines the efficacy of anti-EGFR mAbs in relation to primary tumor sidedness (PTS).
Patient and methods
This pooled analysis was carried out using individual patient data from five randomized studies in the first-line setting of mCRC. The population of interest was limited to patients with BRAFmt mCRC and known PTS. For analysis, treatment was stratified into two groups: those treated with anti-EGFR mAbs and those without. Dichotomous variables, such as overall response rate and objective response rate (ORR), were compared using chi-square or Fisher’s exact test. Time-to-event endpoints [progression-free survival (PFS) and overall survival (OS)] were analyzed using the Kaplan–Meier method, log-rank test, and Cox regression. An interaction test was carried out via Cox regression.
Results
A total of 102 patients with BRAFmt mCRC were identified. The type of targeted therapy (anti-EGFR-based versus non-anti-EGFR) did not significantly impact the outcome. However, in patients with left-sided primary tumors, anti-EGFR mAb-based treatment, compared with non-anti-EGFR, was associated with a higher ORR (58% versus 34%; P < 0.01), trended toward improved PFS [hazard ratio (HR) 0.62; 95% confidence interval (CI) 0.34-1.13; P = 0.12], and demonstrated prolonged OS (HR 0.38; 95% CI 0.20-0.72; P < 0.01). In patients with right-sided primary tumors, anti-EGFR-based therapy had no effect on ORR (33% versus 36%; P > 0.99), induced inferior PFS (HR 1.97; 95% CI 1.12-3.47; P = 0.02), and trended toward a worse OS (HR 1.76; 95% CI 0.99-3.13; P = 0.05).
Conclusion
This analysis suggests that PTS has predictive value for the efficacy of anti-EGFR mAb in the first-line treatment of BRAFmt mCRC.
Key words: BRAF mutation, EGFR antibody, metastatic colorectal cancer, primary tumor location, primary tumor sidedness
Highlights
-
•
PTS has no prognostic impact on patients with BRAFmt mCRC.
-
•
The prognostic impact of PTS may vary between sexes in BRAFmt mCRC.
-
•
Patients with BRAFmt mCRC and left-sided primaries may benefit from first-line anti-EGFR-based therapy.
Background
Initial systemic treatment of metastatic colorectal cancer (mCRC) is based on the tumor’s molecular biology. Upfront testing presently includes not only RAS and BRAF mutational status, but also analyses of DNA mismatch repair (MMR) or microsatellite instability-high (MSI-H) status. While agents directed against the vascular endothelial growth factor (VEGF) can be used regardless of RAS mutation status, agents directed against the epidermal growth factor receptor (EGFR) are not effective in RAS-mutated tumors. They are therefore restricted to RAS wild-type mCRC.1
In mCRC, BRAFV600E mutations occur at a rate of 8%-10%.2, 3, 4 BRAFV600E and RAS mutations are nearly always mutually exclusive.4,5 Patients with proficient MMR and BRAFV600E-mutated mCRC typically show a poor prognosis and survival times remain in the range of 11-19 months in most studies.6, 7, 8, 9
According to a recent meta-analysis of the GONO group, FOLFOX (or FOLFIRI) plus bevacizumab can be regarded as the recommended first-line standard in BRAFV600E-mutated mCRC, while no increased benefit was observed in this subgroup when the more intensive triplet regimen FOLFOXIRI was applied in combination with bevacizumab.10
Although bevacizumab is established in the treatment of BRAFV600E-mutated mCRC, this is not the case for anti-EGFR monoclonal antibodies (mAbs). In fact, there is an ongoing controversial debate about whether anti-EGFR agents are not only ineffective but may even be harmful in this subgroup.11,12 In the recently published FIRE-4.5 study, FOLFOXIRI combined with anti-EGFR mAb was inferior to FOLFOXIRI in combination with bevacizumab.13
A more differentiated analysis of a potentially complex situation was made possible by a subgroup evaluation of FIRE-3, where patients with BRAFV600E-mutated mCRC were evaluated according to response dynamics.9 Early tumor shrinkage (ETS) was achieved in 53% in the cetuximab arm and 33% in the bevacizumab arm. ETS compared with no ETS was associated with a favorable outcome [overall survival (OS) 29.8 versus 5.9 months] in cetuximab-treated, but not in bevacizumab-treated patients (11.8 months versus 13.7 months).9 This analysis demonstrates that BRAFV600E-mutated mCRC is a heterogeneous disease with distinctly different patterns of response to anti-EGFR and anti-VEGF agents. The heterogeneity of BRAFV600E-mutated mCRC has been well established by Barras et al.3 and Guinney et al.14 describing distinct molecular subgroups within the population of patients with BRAFV600E-mutated mCRC according to their gene expression profile. Those subtypes differ in their prognosis and potentially in the efficacy of treatments.
The present analysis targets the biological heterogeneity of BRAFV600E-mutated mCRC with a specific focus on primary tumor sidedness (PTS). To increase the strength of the analysis, individual patient data from five randomized first-line studies were included.
Methods
Trials
The present analysis includes individual patient data from five randomized prospective AIO trials (FIRE-1, CIOX, FIRE-3, XELAVIRI, and VOLFI) carried out in the first-line treatment setting of mCRC. All trials were conducted according to the Declaration of Helsinki and were approved by ethics committees. Detailed reports of all trials have been published previously.9,15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103677, provides an overview of the included studies.
Patients
A pseudonymized clinical database of the trials with the preselection of BRAFV600E-mutant/RAS wild-type disease was established including the following information for each patient: trial, treatment arm, use of EGFR antibody, age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, tumor characteristics (primary tumor sidedness and metastatic sites), and prior adjuvant treatment. Tumor samples assigned to each patient were tested for mutational status (RAS and BRAF) as described previously.9,17, 18, 19, 20, 21, 22, 23,25,26
Primary tumor sidedness and location
Information on primary tumor sidedness was extracted from the respective study report forms. Primary tumors were classified as right- versus left-sided mCRC with a cut-off at the splenic flexure. Patients with more than one primary tumor were excluded from the analysis.
Treatment
Treatment procedures were described in previous publications and are summarized in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103677.9,17, 18, 19, 20, 21, 22, 23,25,26 For further analysis, treatment was stratified into two groups: treatment with or without anti-EGFR antibody. The latter group comprises chemotherapy regimens with or without the VEGF inhibitor bevacizumab.9,17, 18, 19, 20, 21, 22, 23,25,26
Definition of efficacy endpoints
Objective response rate (ORR) was evaluated according to the World Health Organization (WHO) classifications (FIRE-1), RECIST 1.0 (CIOX, FIRE-3), or RECIST 1.1 (XELAVIRI, VOLFI). Progression-free survival (PFS) was defined as the time from randomization to the first progression of disease or death from any cause. In addition, the XELAVIRI study defined PFS as time from randomization to failure of strategy, meaning switching to an anticancer drug not included in the XELAVIRI study. OS was defined as time from randomization until death from any cause.
Statistical analysis
All statistical analyses were carried out using SAS software (version 9.4; SAS Institute, Cary, NC) and SPSS version 28.0 software (IBM Corporation, Armonk, NY). Survival was expressed as medians including 95% confidence intervals (CIs) by the Kaplan–Meier method and compared using log-rank tests. In addition, Cox regression analyses with maximum-likelihood estimation were used for interaction testing. Dichotomous variables were compared by Fisher’s exact test or the chi-square test. Odds ratios were indicated when appropriate with 95% CIs. The two-sided significance level was set to 0.05 and estimates are reported with 95% CI.
Results
Population
Out of the five trials, 1393 patients with known mutational status were identified. A total of 102 patients (7.3%) had BRAFV600E-mutant/RAS wild-type (BRAFmt) tumors with known primary tumor location. Patients with more than one primary tumor were excluded from the analysis. Please also refer to Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103677.
Patient and tumor characteristics
The baseline characteristics of the 102 patients with BRAFmt mCRC are summarized in Table 1. In this population, 55 patients (54%) presented with right-sided primary tumors (RSPTs) and 47 (46%) with left-sided primary tumors (LSPTs). PTS was statistically significantly associated with sex. Patients with RSPT were more likely to be female (P = 0.04).
Table 1.
Patient and tumor characteristics of BRAFV600E mutant/RAS wild-type population according to primary tumor sidedness
| Characteristics | BRAFmt population (n = 102), n (%) | Left-sided primaries (n = 47), n (%) | Right-sided primaries (n = 55), n (%) |
|---|---|---|---|
| Study | |||
| FIRE-1 | 5 (5) | 3 (6) | 2 (4) |
| CIOX | 17 (17) | 6 (13) | 11 (20) |
| FIRE-3 | 47 (46) | 23 (49) | 24 (44) |
| XELAVIRI | 19 (19) | 9 (19) | 10 (18) |
| VOLFI | 14 (14) | 6 (13) | 8 (15) |
| Sex | |||
| Male | 56 (55) | 31 (66) | 25 (45) |
| Female | 46 (45) | 16 (34) | 30 (55) |
| Age (years) | |||
| ≤70 | 77 (75) | 38 (81) | 39 (71) |
| >70 | 25 (25) | 9 (19) | 16 (29) |
| ECOG | |||
| 0 | 53 (52) | 21 (45) | 32 (58) |
| 1 | 47 (46) | 25 (53) | 22 (40) |
| 2 | 2 (2) | 1 (2) | 1 (2) |
| Antibody | |||
| None | 13 (13) | 7 (15) | 6 (11) |
| Anti-EGFR | 46 (45) | 21 (45) | 25 (45) |
| Anti-VEGF | 43 (42) | 19 (40) | 24 (44) |
| Metastatic spread | |||
| Liver | 72 (71) | 30 (64) | 42 (76) |
| Liver-limited | 25 (25) | 11 (23) | 14 (25) |
| Lung | 29 (28) | 15 (32) | 14 (25) |
| Lymph nodes | 51 (50) | 21 (45) | 30 (55) |
| Peritoneum | 19 (19) | 9 (19) | 10 (18) |
| Number of metastatic sites | |||
| 1 | 36 (35) | 19 (40) | 17 (27) |
| ≥2 | 56 (55) | 23 (49) | 33 (60) |
| Unknown | 10 (10) | 5 (11) | 5 (9) |
| Onset of metastases | |||
| Synchronous | 55 (54) | 23 (49) | 32 (58) |
| Metachronous | 15 (15) | 12 (26) | 3 (5) |
| Unknown | 32 (31) | 12 (26) | 20 (36) |
| Previous chemotherapy | |||
| No | 86 (84) | 38 (81) | 48 (87) |
| Yes | 15 (15) | 9 (19) | 6 (11) |
| Unknown | 1 (1) | 0 (0) | 1 (2) |
BRAFmt, BRAFV600E mutant/RAS wild-type.
Prognostic impact of primary tumor sidedness
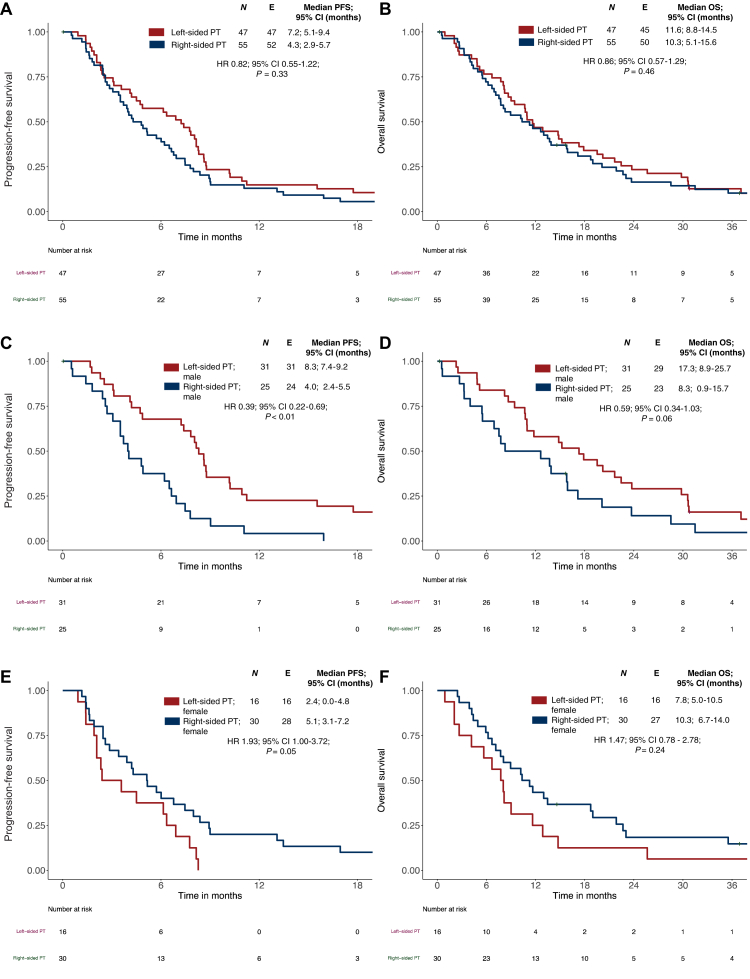
In the overall population of BRAFmt mCRC, ORR was superior in patients with LSPT compared with RSPT without being statistically significant (LSPT: 55% versus RSPT: 35%; P = 0.05). Numerically more favorable data were obtained in LSPT with regard to PFS (LSPT: 7.2 months versus RSPT: 4.3 months; hazard ratio (HR) 0.82; 95% CI 0.55-1.22; P = 0.33) and OS (LSPT: 11.6 months versus RSPT: 10.3 months; HR 0.86; 95% CI 0.57-1.29; P = 0.46; see Figure 1A and B).
Figure 1.
Progression-free survival (PFS) and overall survival (OS) according to primary tumor sidedness and sex in BRAFmt metastatic colorectal cancer (mCRC). (A) PFS according to primary tumor sidedness in BRAFmt mCRC. (B) OS according to primary tumor sidedness in BRAFmt mCRC. (C) PFS in male patients with BRAFmt mCRC according to primary tumor sidedness. (D) OS in male patients with BRAFmt mCRC primary tumor sidedness. (E) PFS in female patients with BRAFmt mCRC according to primary tumor sidedness. (F) OS in female patients with BRAFmt mCRC according to primary tumor sidedness. P values correspond to Cox regression. BRAFmt, BRAFV600E mutant/RAS wild-type; CI, confidence interval; HR, hazard ratio; OS, overall survival; PT, primary tumor.
The multivariate analysis for OS in LSPT revealed a significant association with sex (P = 0.01); however, in RSPT no significant association was found. To better understand the impact on outcome according to PTS and sex, we analyzed the outcome in LSPT and RSPT according to sex:
In male patients, LSPT compared with RSPT was associated with superior outcome parameters: there was a trend regarding ORR (LSPT: 71% versus RSPT: 45%; P = 0.09), a statistically relevant improvement of PFS (HR 0.39; 95% CI 0.22-0.69; P < 0.01), and clinically meaningful trend in OS (HR 0.59; 95% CI 0.34-1.03; P = 0.06).
A reverse pattern was observed in female patients. ORR was comparable, regardless of sidedness (LSPT: 25% versus RSPT: 27%, P > 0.99), but PFS (HR 1.93; 95% CI 1.00-3.72; P = 0.05) and OS rather favored RSPT without being statistically relevant (HR 1.47; 95% CI 0.78-2.78; P = 0.24). The Kaplan–Meier curves are indicated in Figure 1C–F.
Data obtained in BRAFmt mCRC suggest that LSPT is associated with better outcomes in male, but not in female patients.
Predictive value of sidedness of primary tumor
Of 102 patients with BRAFmt mCRC, 45% (n = 46) received anti-EGFR-based first-line therapy, while 55% (n = 56) did not. In Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103677, baseline patient and tumor characteristics of the treatment groups are shown.
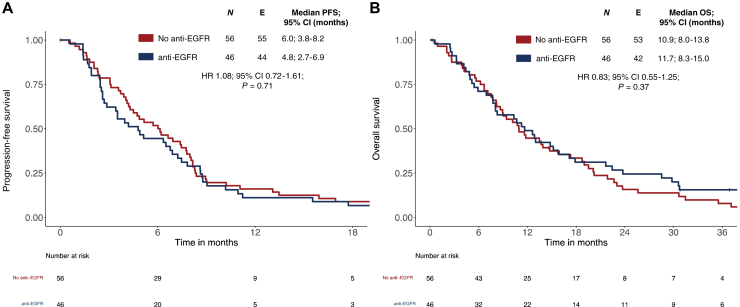
ORR in the BRAFmt patients was higher in patients treated with anti-EGFR-based therapy (58% versus 34%; P = 0.02). Analysis of the overall population showed comparable PFS and OS independent of having received anti-EGFR agents or not (see Figure 2A and B).
Figure 2.
Progression-free survival (PFS) and overall survival (OS) in BRAFmt metastatic colorectal cancer (mCRC) cases when treated with or without anti-EGFR. (A) PFS according to treatment with or without anti-EGFR in BRAFmt mCRC. (B) OS according to treatment with or without anti-EGFR in BRAFmt mCRC. P values correspond to Cox regression. BRAFmt, BRAFV600E mutant/RAS wild-type; CI, confidence interval; EGFR, epidermal growth factor receptor; HR, hazard ratio.
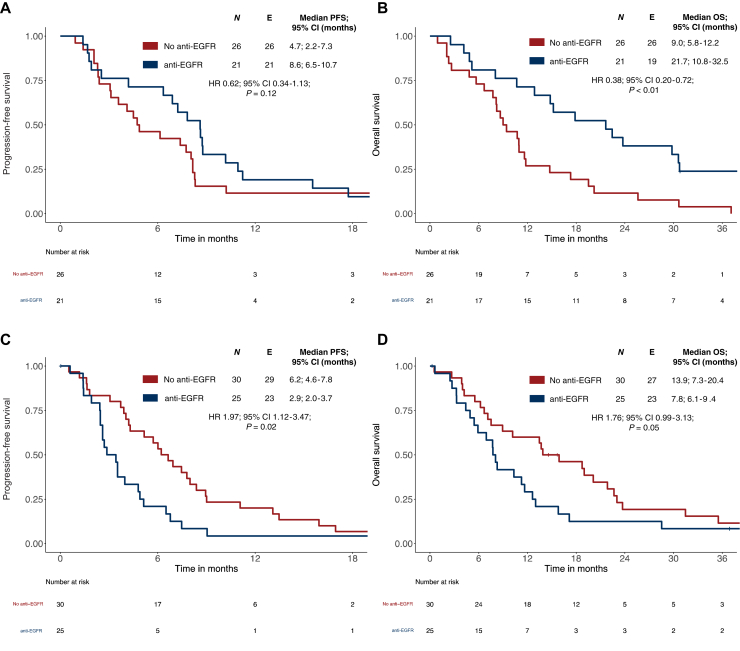
In LSPT patients with BRAFmt tumors, anti-EGFR mAb-based treatment versus none was associated with higher ORR (81% versus 35%; P < 0.01), more favorable OS (HR 0.38; 95% CI 0.20-0.72; P < 0.01) and a trend toward improved PFS (HR 0.62; 95% CI 0.34-1.13; P = 0.12).
By contrast, this effect was not observed in patients with RSPT with BRAFmt tumors. In this subgroup, anti-EGFR-based therapy had no positive impact on ORR (36% versus 33%; P > 0.99), induced inferior results with regard to PFS (HR 1.97; 95% CI 1.12-3.47; P = 0.02), and trended toward worse OS (HR 1.76; 95% CI 0.99-3.13; P = 0.05). The respective Kaplan–Meier curves are indicated in Figure 3A–D.
Figure 3.
Progression-free survival (PFS) and overall survival (OS) in BRAFmt metastatic colorectal cancer (mCRC) cases according to primary tumor sidedness and treatment with or without anti-EGFR. (A) PFS in left-sided primary tumor (LSPT) according to treatment with or without anti-EGFR in BRAFmt mCRC. (B) OS in LSPT according to treatment with or without anti-EGFR in BRAFmt mCRC. (C) PFS in right-sided primary tumor (RSPT) according to treatment with or without anti-EGFR in BRAFmt mCRC. (D) OS in RSPT according to treatment with or without anti-EGFR in BRAFmt mCRC. P values correspond to Cox regression. BRAFmt, BRAFV600E mutant/RAS wild-type; CI, confidence interval; EGFR, epidermal growth factor receptor; HR, hazard ratio.
With regard to OS, the interaction of sidedness and anti-EGFR antibody efficacy was also present when the analysis was restricted to patients with BRAFV600E-mutated mCRC from FIRE-3 and VOLFI. These studies represent the purest population as they contained direct randomization of anti-EGFR-based treatment versus no antibody or bevacizumab (P < 0.01). The Kaplan–Meier curves of the FIRE-3/VOLFI subset are shown in Supplementary Figure S2A and B, available at https://doi.org/10.1016/j.esmoop.2024.103677.
An interaction test revealed significant results for OS and showed a trend toward significance for PFS between primary tumor sidedness and the treatment arms in the respective study (OS: P = 0.01; PFS: P < 0.01); additionally, a significant interaction was observed between anti-EGFR and non-anti-EGFR treatment and PTS for both OS (P < 0.010) and PFS (P = 0.05). The interaction test for OS was significant between anti-EGFR and non-anti-EGFR treatments and PTS when the analysis was restricted to patients from the VOLFI and FIRE-3 studies (n = 61, P = 0.03).
Discussion
The presented analysis, based on five randomized trials including data from 102 patients with BRAFmt mCRC out of 1393 patients with known molecular subtype, represents an adequate basis for evaluating the prognostic and predictive effects of the primary tumor’s sidedness in BRAFmt mCRC. However, it is important to clarify that the data come from five pooled trials, each using different therapeutic strategies with various chemotherapy regimens and antibody usage. Currently, some of these regimens are no longer considered standard of care for this population.
In our analysis, patients with BRAFmt mCRC with LSPTs and RSPTs showed no difference in PFS and OS overall.
Interestingly, primary tumor sidedness predicted the efficacy of anti-EGFR antibody therapy in our BRAFmt mCRC cohort in terms of ORR and OS. LSPTs appeared to benefit from anti-EGFR therapy, whereas RSPTs did not. However, anti-EGFR antibodies did not affect PFS in our cohort. Whether PFS is a good marker for defining the efficacy of anti-EGFR mAb has been questioned in previous publications,27,28 and it has been suggested that anti-EGFR antibodies may instead impact ORR and OS.19,23,29, 30, 31 Similar combinations with increased ORR and prolonged OS, even in the absence of clinically relevant effects on PFS, have been frequently observed in an evidently anti-EGFR-sensitive population (i.e. RAS/BRAF wild-type mCRC).23,32, 33, 34 The stronger beneficial effect on ORR compared with OS for anti-EGFR antibody treatment could stem from rapidly developing resistance to anti-EGFR medication in BRAFmt mCRC. Therefore it could be hypothesized that the presence of BRAFV600E mutations may not necessarily predict a lack of anti-EGFR antibody efficacy. Our findings suggest that patients with BRAFmt mCRC originating from LSPTs may benefit from anti-EGFR antibodies to a similar extent as those with RAS/BRAF wild-type tumors. This raises the question of whether there are patients with left-sided BRAFmt mCRC who could benefit from anti-EGFR-containing first-line therapy, especially when combined with BRAF inhibition.
However, it should be noted that this pooled analysis is limited by its retrospective and exploratory design, as well as by the low number of patients, which restricts the ability to draw definite conclusions. Further, there may be confounding aspects influencing these results. In particular, the missing information on mutational status in the analyzed studies (27%, see Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103677) and the overall low prevalence of BRAF mutations in mCRC may introduce bias.
The metastatic spread of right-sided tumors compared with left-sided ones may also introduce bias. In our analysis, left-sided tumors had a higher likelihood of having only one metastatic side and of involving pulmonary metastases, thus indicating a lower overall tumor load. This could disadvantage patients with RSPTs. In addition, patients with LSPTs were younger, potentially leading to a healthier patient population and a higher percentage of second-line therapies, which might account for the discordance in PFS and OS results. However, this does not explain the higher ORR observed with anti-EGFR treatment.
However, patients treated with anti-EGFR-containing regimens were from more recent studies, whereas 27% of the study population treated with non-anti-EGFR regimens were part of the FIRE-1 (mIROX) and XELAVIRI (sequential treatment option, see Supplementary Table S1 and S2, available at https://doi.org/10.1016/j.esmoop.2024.103677). These therapeutic options are now considered suboptimal and may also contribute to bias.
Thus the presented results might be secondary to the generally better prognosis of left-sided tumors, which could be more pronounced in the optimally treated patient population. Nevertheless, the interaction test for OS between primary tumor sidedness and the applied treatment (including respective treatment arms and anti-EGFR versus non-anti-EGFR containing therapy) was positive.
The applied second-line therapies could introduce bias, potentially distorting OS.
Furthermore, pooling data from five studies, two of which directly randomized anti-EGFR antibodies and each using different treatment regimens, may have introduced potential undetected biases due to the heterogeneity of the populations.
Our findings contrast with reports identifying classical BRAFV600E mutations as likely negative predictors of anti-EGFR-directed therapy in chemotherapy-treated cohorts.11,35,36 However, it could be argued that these studies did not adjust their analyses for sidedness and might therefore be biased by the relative over-representation of BRAFV600E mutations in right-sided colon segments.4
Although the FIRE-4.5 trial demonstrated a detrimental effect of anti-EGFR antibodies when combined with FOLFOXIRI, RSPTs appear to gain benefit more from FOLFOXIRI combined with bevacizumab regarding ORR, PFS, and OS. However, this effect was not observed in LSPTs.13 In the FIRE-4.5 trial, the triplet chemotherapy FOLFOXIRI was used as the backbone, whereas this analysis primarily includes patients treated with doublet chemotherapy. Thus any additional lack of benefit derived from adding anti-EGFR in LSPTs could be partially linked to the chemotherapy backbone, similar to RAS/BRAF wild-type tumors.37 Even if this hypothesis is valid, this analysis does not address whether adding anti-EGFR in the first-line setting would impact the efficacy of the approved second-line treatment with encorafenib and cetuximab. Consecutively, current guideline recommendations38 do not recommend anti-EGFR-targeted antibody treatment as part of first-line therapy, with cetuximab currently being used primarily in combination with a BRAF inhibitor for refractory patients.39
Moreover, if the BREAKWATER trial, which examines the role of combining anti-BRAF and anti-EGFR treatments with chemotherapy in the first-line setting, yields positive results, then this analysis may become less relevant.
As already mentioned, there is a vast clinical heterogeneity among patients with BRAFmt mCRC.3,14 Therefore to better predict the individual disease courses, clinical scoring systems with prognostic significance have been developed.40 Several molecular factors contributing to this heterogeneity have been described: a higher plasmatic BRAFV600E allele frequency is associated with more aggressive disease and worse outcomes, likely due to the associated higher tumor load.41 In addition, some distinct genomic alterations, such as RNF43 mutations, have been identified as having prognostic and predictive impacts with regard to the efficacy of systemic and/or targeted therapies.42 Furthermore, MSI status is a crucial prognostic and predictive marker in the era of immunotherapy for patients with mCRC. Patients with BRAFmt MSI-H mCRC have significantly worse outcomes when treated with chemotherapy compared with immunotherapy.43,44 MSI-H tumors occur in ∼21% of BRAFmt mCRC cases and are more frequently found in right-sided tumors, which could be a relevant negative confounder in this chemotherapy-treated cohort, especially because MSI status is missing.45 In addition, different molecular subgroups could explain the varied prognostic impact of primary tumor sidedness between sexes, which could remain undetected in this analysis.
Nevertheless, primary tumor sidedness is a known prognostic marker in RAS/BRAF wild-type mCRC, serving as a surrogate of underlying molecular alterations. The ease of determining primary tumor sidedness is an advantage and could have clinical significance in BRAFmt mCRC.
The results of our analysis should be interpreted as hypothesis-generating and need confirmation from further trials with direct anti-EGFR antibody randomization, such as CRYSTAL, PRIME, OPUS, CALGB/SWOG 80405, and PEAK.46, 47, 48, 49 These trials should pay special attention to microsatellite status, prognostic groups, and sex.
Conclusions
In our analysis, patients with BRAFV600E-mutant, RAS wild-type mCRC with left-sided primaries had a numerically better prognosis, and a beneficial effect from first-line anti-EGFR-based therapy is suspected. Moreover, the prognostic impact of primary tumor sidedness may differ between sexes.
Acknowledgements
The authors thank all participating patients and their loved ones, all study investigators, and their staff.
Funding
This work was supported by the hospital of the university (LMU) which was the legal sponsor of FIRE-1 (supported by Pfizer), CIOX (supported by Merck-Darmstadt; Roche and Pfizer), FIRE-3 (supported by Merck-Darmstadt and Pfizer), and XELAVIRI (supported by Roche) (no grant number). The AIO Studien gGmbH was the legal sponsor of VOLFI (supported by Amgen). The supporting parties had no role in the design, analyses, or decision to submit the data for publication.
Disclosure
AHSA reports honoraria from MSD, Servier, Merck KGaA, MSD, Pfizer, Pierre-Fabre, Roche, Amgen, and BMS; travel, accommodations, and expenses covered by Nordic, Servier, Merck KGaA, MSD, Pfizer, Pierre-Fabre, Roche, and Amgen; performing consulting or advisory roles for Roche and MSD. VH reports honoraria from Merck, Amgen, Roche, Sanofi, Servier, Pfizer, Pierre-Fabre, AstraZeneca, BMS, MSD, Novartis, Boehringer Ingelheim, Celgene, SIRTEX, Terumo, OncoSil, NORDIC, Seagen, and GSK; performing consulting or advisory roles for Merck, Amgen, Roche, AstraZeneca, Sanofi, Boehringer Ingelheim, Celgene, Sirtex Medical, Baxalta, Servier, Halozyme, MSD, BMS, GSK, Nordic, and Jansen; research funding from Merck (to institute), Amgen (to institute), Roche (to institute), Celgene (to institute), Boehringer Ingelheim (to institute), Sirtex Medical (to institute), and Shire (to institute); travel, accommodations, and expenses covered by Merck, Roche, Sirtex Medical, Amgen, Servier, Shire, MSD, BMS, and Nordic. SS reports honoraria from Amgen, AstraZeneca, Bayer, BMS, Daichi-Sanyko, ESAI, Leo-Pharma, Lilly, Merck KGaA, MSD, Pierre-Fabre, Roche, Sanofi, Servier, Taiho, and Takeda; performing consulting or advisory roles for Amgen, AstraZeneca, Bayer, BMS, Daiichi Sankyo, ESAI, Lilly, Merck KGaA, MSD, Pierre-Fabre, Roche, Sanofi, Servier, Taiho, and Takeda; research funding from Merck KGaA, Pierre-Fabre, Roche, and Servier. DPM reports honoraria from Amgen, AstraZeneca, Servier, BMS, Taiho Pharmaceutical, MSD, Pierre-Fabre, Onkowissen, Sanofi, Lilly, Incyte, Takeda, Merck Serono, Regeneron, Rottapharm, and G1; performing consulting or advisory roles for MSD, Roche, Servier, Incyte, BMS, Pierre-Fabre, Lilly, Cor2Ed, IQVIA, Onkowissen, Merck Serono, and Amgen; research funding from Amgen and Servier; travel support/expenses covered by Amgen, Merck Serono, and Servier. KH reports honoraria from Roche, Taiho, BMS, Merck, and streamedup! performing consulting or advisory roles for Servier, MSD (institutional), Merck, and Janssen; travel support/expenses covered by Amgen, Merck, and Servier. AS reports honoraria from Roche, Servier, Taiho Pharmaceutical, Merck KGaA, Takeda, and Amgen; travel, accommodations, and expenses covered by Amgen, Roche, Lilly Oncology, and Pfizer; and performing consulting or advisory roles for BMS, Novocure, and Takeda. AT reports honoraria from BNT and Roche. CGJ reports honoraria/travel support from GSK, Roche, Grünenthal, Elsevier, and Lilly. AJ reports honoraria from Amgen, AstraZeneca, Bayer Pharmaceuticals, BMS, Boehringer Ingelheim, Merck KGaA, Lilly, MSD, Novartis, Roche Pharma, Stemline, and Takeda; performing consulting or advisory roles for Amgen, AstraZeneca, Biocartis, QuIP GmbH, and Roche Pharma. LW reports receiving honoraria from/serving on the advisory boards of Roche and Servier; and travel, accommodations, and expenses covered by Amgen. All other authors have declared no conflicts of interest.
Data sharing
The datasets used during this analysis are available from the corresponding author on reasonable request.
Supplementary data
References
- 1.Motta R., Cabezas-Camarero S., Torres-Mattos C., et al. Personalizing first-line treatment in advanced colorectal cancer: present status and future perspectives. J Clin Transl Res. 2021;7(6):771–785. [PMC free article] [PubMed] [Google Scholar]
- 2.Davies H., Bignell G.R., Cox C., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 3.Barras D., Missiaglia E., Wirapati P., et al. BRAF V600E mutant colorectal cancer subtypes based on gene expression. Clin Cancer Res. 2017;23(1):104–115. doi: 10.1158/1078-0432.CCR-16-0140. [DOI] [PubMed] [Google Scholar]
- 4.Tran B., Kopetz S., Tie J., et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117(20):4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqui A.D., Piperdi B. KRAS mutation in colon cancer: a marker of resistance to EGFR-I therapy. Ann Surg Oncol. 2010;17(4):1168–1176. doi: 10.1245/s10434-009-0811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modest D.P., Ricard I., Heinemann V., et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. 2016;27(9):1746–1753. doi: 10.1093/annonc/mdw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cremolini C., Loupakis F., Antoniotti C., et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E., Kohne C.H., Lang I., et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 9.Stintzing S., Miller-Phillips L., Modest D.P., et al. Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: analysis of the FIRE-3 (AIO KRK-0306) study. Eur J Cancer. 2017;79:50–60. doi: 10.1016/j.ejca.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Cremolini C., Antoniotti C., Stein A., et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol. 2020 doi: 10.1200/JCO.20.01225. [DOI] [PubMed] [Google Scholar]
- 11.Pietrantonio F., Petrelli F., Coinu A., et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51(5):587–594. doi: 10.1016/j.ejca.2015.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Rowland A., Dias M.M., Wiese M.D., et al. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br J Cancer. 2015;112(12):1888–1894. doi: 10.1038/bjc.2015.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stintzing S., Heinrich K., Tougeron D., et al. FOLFOXIRI plus cetuximab or bevacizumab as first-line treatment of BRAF(V600E)-mutant metastatic colorectal cancer: the randomized phase II FIRE-4.5 (AIO KRK0116) study. J Clin Oncol. 2023;41(25):4143–4153. doi: 10.1200/JCO.22.01420. [DOI] [PubMed] [Google Scholar]
- 14.Guinney J., Dienstmann R., Wang X., et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modest D.P., Schulz C., von Weikersthal L.F., et al. Outcome of patients with metastatic colorectal cancer depends on the primary tumor site (midgut vs. hindgut): analysis of the FIRE1-trial (FuFIRI or mIROX as first-line treatment) Anticancer Drugs. 2014;25(2):212–218. doi: 10.1097/CAD.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 16.Fischer von Weikersthal L., Schalhorn A., Stauch M., et al. Phase III trial of irinotecan plus infusional 5-fluorouracil/folinic acid versus irinotecan plus oxaliplatin as first-line treatment of advanced colorectal cancer. Eur J Cancer. 2011;47(2):206–214. doi: 10.1016/j.ejca.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Modest D.P., Fischer von Weikersthal L., Decker T., et al. Sequential versus combination therapy of metastatic colorectal cancer using fluoropyrimidines, irinotecan, and bevacizumab: a randomized, controlled study-XELAVIRI (AIO KRK0110) J Clin Oncol. 2019;37(1):22–32. doi: 10.1200/JCO.18.00052. [DOI] [PubMed] [Google Scholar]
- 18.Modest D.P., Jung A., Moosmann N., et al. The influence of KRAS and BRAF mutations on the efficacy of cetuximab-based first-line therapy of metastatic colorectal cancer: an analysis of the AIO KRK-0104-trial. Int J Cancer. 2012;131(4):980–986. doi: 10.1002/ijc.26467. [DOI] [PubMed] [Google Scholar]
- 19.Modest D.P., Martens U.M., Riera-Knorrenschild J., et al. FOLFOXIRI plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: the randomized, open-label, phase II VOLFI study (AIO KRK0109) J Clin Oncol. 2019;37(35):3401–3411. doi: 10.1200/JCO.19.01340. [DOI] [PubMed] [Google Scholar]
- 20.Moosmann N., von Weikersthal L.F., Vehling-Kaiser U., et al. Cetuximab plus capecitabine and irinotecan compared with cetuximab plus capecitabine and oxaliplatin as first-line treatment for patients with metastatic colorectal cancer: AIO KRK-0104--a randomized trial of the German AIO CRC study group. J Clin Oncol. 2011;29(8):1050–1058. doi: 10.1200/JCO.2010.31.1936. [DOI] [PubMed] [Google Scholar]
- 21.Stahler A., Heinemann V., Giessen-Jung C., et al. Influence of mRNA expression of epiregulin and amphiregulin on outcome of patients with metastatic colorectal cancer treated with 5-FU/LV plus irinotecan or irinotecan plus oxaliplatin as first-line treatment (FIRE 1-trial) Int J Cancer. 2016;138(3):739–746. doi: 10.1002/ijc.29807. [DOI] [PubMed] [Google Scholar]
- 22.Stintzing S., Fischer von Weikersthal L., Decker T., et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer—subgroup analysis of patients with KRAS: mutated tumours in the randomised German AIO study KRK-0306. Ann Oncol. 2012;23(7):1693. doi: 10.1093/annonc/mdr571. 1639. [DOI] [PubMed] [Google Scholar]
- 23.Stintzing S., Modest D.P., Rossius L., et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;17(10):1426–1434. doi: 10.1016/S1470-2045(16)30269-8. [DOI] [PubMed] [Google Scholar]
- 24.Heinemann V., von Weikersthal L.F., Decker T., et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 25.Heinemann V., von Weikersthal L.F., Decker T., et al. FOLFIRI plus cetuximab or bevacizumab for advanced colorectal cancer: final survival and per-protocol analysis of FIRE-3, a randomised clinical trial. Br J Cancer. 2021;124(3):587–594. doi: 10.1038/s41416-020-01140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volz N.B., Hanna D.L., Stintzing S., et al. Polymorphisms within immune regulatory pathways predict cetuximab efficacy and survival in metastatic colorectal cancer patients. Cancers (Basel) 2020;12(10):2947. doi: 10.3390/cancers12102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venook A.P., Tabernero J. Progression-free survival: helpful biomarker or clinically meaningless end point? J Clin Oncol. 2015;33(1):4–6. doi: 10.1200/JCO.2014.57.9557. [DOI] [PubMed] [Google Scholar]
- 28.Giessen C., Laubender R.P., Ankerst D.P., et al. Progression-free survival as a surrogate endpoint for median overall survival in metastatic colorectal cancer: literature-based analysis from 50 randomized first-line trials. Clin Cancer Res. 2013;19(1):225–235. doi: 10.1158/1078-0432.CCR-12-1515. [DOI] [PubMed] [Google Scholar]
- 29.Khattak M.A., Martin H., Davidson A., Phillips M. Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: a meta-analysis of randomized clinical trials. Clin Colorectal Cancer. 2015;14(2):81–90. doi: 10.1016/j.clcc.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Piessevaux H., Buyse M., Schlichting M., et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2013;31(30):3764–3775. doi: 10.1200/JCO.2012.42.8532. [DOI] [PubMed] [Google Scholar]
- 31.Douillard J.Y., Siena S., Peeters M., Koukakis R., Terwey J.H., Tabernero J. Impact of early tumour shrinkage and resection on outcomes in patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer. 2015;51(10):1231–1242. doi: 10.1016/j.ejca.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Arnold D., Lueza B., Douillard J.Y., et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713–1729. doi: 10.1093/annonc/mdx175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holch J.W., Ricard I., Stintzing S., Modest D.P., Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98. doi: 10.1016/j.ejca.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Tejpar S., Stintzing S., Ciardiello F., et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2017;3:194–201. doi: 10.1001/jamaoncol.2016.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Nicolantonio F., Martini M., Molinari F., et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26(35):5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 36.De Roock W., Claes B., Bernasconi D., et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 37.Cremolini C., Rossini D., Lonardi S., et al. Modified FOLFOXIRI plus panitumumab (mFOLFOXIRI/PAN) versus mFOLFOX6/PAN as initial treatment of patients with unresectable RAS and BRAF wild-type metastatic colorectal cancer (mCRC): Results of the phase III randomized TRIPLETE study by GONO. J Clin Oncol. 2022;40(suppl 17) [Google Scholar]
- 38.Cervantes A., Adam R., Rosello S., et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(1):10–32. doi: 10.1016/j.annonc.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Kopetz S., Grothey A., Yaeger R., et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 40.Loupakis F., Intini R., Cremolini C., et al. A validated prognostic classifier for (V600E)BRAF-mutated metastatic colorectal cancer: the ‘BRAF BeCool’ study. Eur J Cancer. 2019;118:121–130. doi: 10.1016/j.ejca.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Ros J., Matito J., Villacampa G., et al. Plasmatic BRAF-V600E allele fraction as a prognostic factor in metastatic colorectal cancer treated with BRAF combinatorial treatments. Ann Oncol. 2023;34(6):543–552. doi: 10.1016/j.annonc.2023.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Elez E., Ros J., Fernandez J., et al. RNF43 mutations predict response to anti-BRAF/EGFR combinatory therapies in BRAF(V600E) metastatic colorectal cancer. Nat Med. 2022;28(10):2162–2170. doi: 10.1038/s41591-022-01976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diaz L.A., Jr., Shiu K.K., Kim T.W., et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. May 2022;23(5):659–670. doi: 10.1016/S1470-2045(22)00197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lenz H.J., Van Cutsem E., Luisa Limon M., et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J Clin Oncol. 2022;40(2):161–170. doi: 10.1200/JCO.21.01015. [DOI] [PubMed] [Google Scholar]
- 45.Venderbosch S., Nagtegaal I.D., Maughan T.S., et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20(20):5322–5330. doi: 10.1158/1078-0432.CCR-14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bokemeyer C., Van Cutsem E., Rougier P., et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48(10):1466–1475. doi: 10.1016/j.ejca.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 47.Douillard J.Y., Oliner K.S., Siena S., et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 48.Schwartzberg L.S., Rivera F., Karthaus M., et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240–2247. doi: 10.1200/JCO.2013.53.2473. [DOI] [PubMed] [Google Scholar]
- 49.Innocenti F., Ou F.S., Qu X., et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J Clin Oncol. 2019;37(14):1217–1227. doi: 10.1200/JCO.18.01798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.