Abstract
The nuclear factor-erythroid 2-related factor 2 (Nrf2) is able to control the redox balance in the cells responding to oxidative damage and other stress signals. The Nrf2 upregulation can elevate the levels of antioxidant enzymes to support against damage and death. In spite of protective function of Nrf2 in the physiological conditions, the stimulation of Nrf2 in the cancer has been in favour of tumorigenesis. Since the dysregulation of molecular pathways and mutations/deletions are common in tumors, Nrf2 can be a promising therapeutic target. The Nrf2 overexpression can prevent cell death in tumor and by increasing the survival rate of cancer cells, ensures the carcinogenesis. Moreover, the induction of Nrf2 can promote the invasion and metastasis of tumor cells. The Nrf2 upregulation stimulates EMT to increase cancer metastasis. Furthermore, regarding the protective function of Nrf2, its stimulation triggers chemoresistance. The natural products can regulate Nrf2 in the cancer therapy and reverse drug resistance. Moreover, nanostructures can specifically target Nrf2 signaling in cancer therapy. The current review discusses the potential function of Nrf2 in the proliferation, metastasis and drug resistance. Then, the capacity of natural products and nanostructures for suppressing Nrf2-mediated cancer progression is discussed.
Keywords: Cancer, Nrf2, cancer drug resistance, cell death, proliferation and metastasis
Introduction
Cancer refers to a large and complex group of diseases that are characterized from normal cells by the uncontrolled growth and spread of abnormal cells throughout the body of the patient [1,2]. It is possible for cancers to affect a wide variety of tissues and organs, increasing its mortality rate [3]. The malignant behaviour of tumor cells has led to their ability in the development of resistance into therapies such as immune evasion [4] and drug resistance [5]. Highlighting the factors involved in the progression of cancer and its development can provide new insights for its treatment, as it has high mortality and morbidity and put heavy socioeconomic costs. Cancer is caused by genetic abnormalities occurring in the DNA [6,7]. Such abnormalities are responsible for impairing the normal regulatory processes of the cells which in turn leads to uncontrolled cell proliferation and division. These mutations can be caused by a wide variety of factors, including a genetic predisposition, exposure to carcinogens including nicotine and UV radiation, and some viral infections. Genetic predisposition is another factor that can play a significant role in the development and induction of these mutations, promoting carcinogenesis. In spite of this, there are a number of cases lacking the track into the specific causes due to the heterogeneous nature of cancer and the involvement of different factors in its progression [8,9]. One of the most distinguishing features of cancer is its propensity to invade neighbouring tissues and, once it has progressed to a more advanced stage, to undergo metastasis, in which it spreads to unreachable parts of the body by way of the circulatory system or the lymphatic system [10,11]. The aggressive behaviour of tumor cells and their invasion into other tissues can affect the normal function, increasing lethality. Until now, multiple kinds of cancers have been identified affecting different parts of body, known as solid and haematological tumors with specific features. Although there are many different types of cancer, the most common manifestations are breast cancer, lung cancer, prostate cancer, and colorectal cancer [12]. The treatment and symptoms are specific for each type of cancer and in order to improve therapy, precision medicine has been introduced [13,14]. The diagnosis of cancer is based on a physical examination, various imaging modalities, and biopsies. This evaluation is vital for the precise classification, extent and location of malignancy. The liquid biopsy [15,16] and nanoparticle-empowered diagnosis [17,18] have been also introduced for the cancer.
More efforts should be made on staging of cancer, as it can determine the severity of disease and is beneficial for directing the future therapies. A number of cancer types are localized in the place of their origin, but other types grow and spread into other parts of body, causing metastasis. The treatment procedure of cancer is based on the cancer type, its stage and patient’s health. Surgical intervention, chemotherapy, radiation treatment, immunotherapy, targeted therapy, and hormone therapy are a number of therapeutic modalities for cancer [19-24]. There have been significant advancements made in the field of precision medicine in recent years, directing better individualized therapies [25,26]. This has resulted improvement in the survival rate of patients. In addition, the preventative measures have been made for the cancer. The lifestyle, smoking, diet and physical activity can affect the process of cancer development. By vaccinations against specific viruses, such as human papillomavirus (HPV) and hepatitis B, the risk of cancer development can be decreased, especially for cervical cancer and head and neck cancer [27-30]. The frequent screenings for cancer including mammograms and colonoscopies, have improved the potential for the early diagnosis of cancer. The knowledge towards aetiology of cancer should be improved and innovative therapeutic methods, and ways for early diagnosis should be a priority. In the recent years, there have been significant progresses in the field of cancer research, especially immunotherapy and targeted treatments [31,32].
In spite of introducing different kinds of therapies for cancer, it is still incurable and therefore, new kinds of therapies should be introduced. The most prominent changes in the tumor cells is the genomic profile alteration. Therefore, novel kinds of therapies should be directed towards the specific targeting of most prominent factors for cancer. The current review focuses on the specific regulation and function of Nrf2 in cancer. The function of Nrf2 in the regulation of proliferation, metastasis, cell death mechanisms and EMT is discussed. Then, the regulation of Nrf2 by non-coding RNAs and the role of Nrf2 in the drug resistance is evaluated. Finally, the modulation of Nrf2 by natural products and nanoparticles for providing new perspectives in cancer therapy is discussed.
Nrf2: basics and signalling
Nuclear factor erythroid 2-related factor 2 (also known as Nrf2) is a transcription factor that belongs to the Cap‘n’Collar (CNC) subfamily of basic leucine zipper (bZip) transcription factors. NRF2 is encoded by the NFE2L2 gene, which is also known as the Nuclear Factor, Erythroid 2 Like 2 gene. Other important members of this CNC subfamily include nuclear factor erythroid-derived 2 (NFE2), nuclear factor RF1, nuclear factor RF2, and nuclear factor RF3. These transcription factors have unique features in the cells, along with the regulation of gene expression [33]. Upon the heterodimer formation, Nrf2 is able to generate coordination with the musculoaponeurotic fibrosarcoma proteins (MAFs) K, G, and F. This provides the capacity to attach to antioxidant response elements and stimulate the transcription of particular genes [34]. The protein Nrf2 is composed of seven different NRF2ECH homology (Neh) domains, all of which have their own unique activities. Neh1 domain’s bZip works with MAFs [34], while Neh2 domain’s two ETGE and DLG motifs exclusively bind to the Kelch domain of Kelch-like ECH-associated protein 1 (KEAP1). In turn, KEAP1 controls the ubiquitination and degradation of NRF2 [34]. The C-terminal Neh3 domain is a transcriptional activation domain interacts with the protein [35]. In addition, Neh4 and Neh5 contribute to transcriptional activation by interacting with CREB-binding protein (CBP), a protein that is active as a histone acetyltransferase [35].
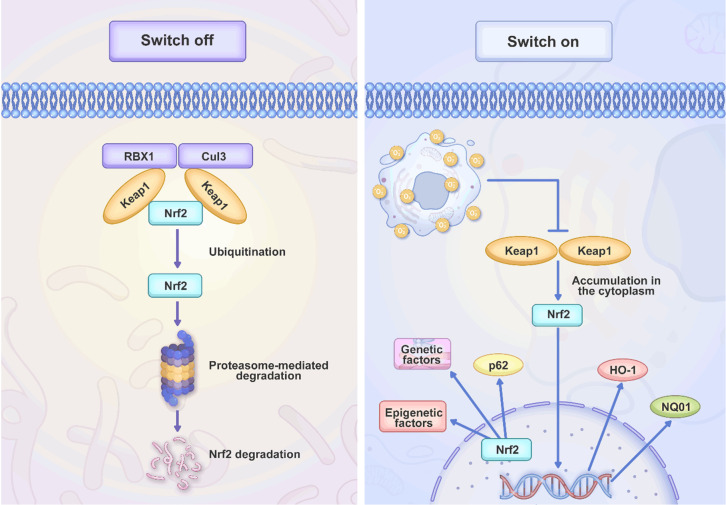
This permits Neh4 and Neh5 to be a part in the process. The Neh6 domain is rich in serine residues and contains two-transducin repeat-containing protein (-TrCP) degrons known as DSGIS and DSAPGS. These degrons are involved in the degradation of Nrf2 [36-39]. Finally, the cytoprotective function of Nrf2 is suppressed resulting from an interaction between the Neh7 domain and the retinoic X receptor alpha (RXRα) [40]. Keap1 is a protein that is comprised of 624 different amino acids and enriched in cysteine; in humans, it contains 27 different cysteine residues. This protein is comprised of five separate domains, including N-terminal region (NTR), tramtrack and Bric-à-Brac (BTB) domain central intervening region (IVR) with a nuclear export signal (NES) accounting for directing Keap1 to the cytoplasm. The C-terminal domain (CTR) is comprised of six Kelch repeats. The BTB domain interacts with Keap1, leading to the formation of homodimers, and it also contributes in the protein association with the cullin-based E3 ligase (Cul3). Due to this association, the Keap1-Cul3-RBX1 (Ring box protein-1) E3 ligase complex is finally generated. On the other hand, Kelch repeats can increase the potential of Keap1 in binding to proteins including Nrf2 and p62 regulating cellular processes (Figure 1) [41].
Figure 1.
An overview of Nrf2 signaling. Nrf2 is a regulator of redox balance and when there is oxidative damage, the stimulation of Nrf2 occurs. This prevents the proteasomal degradation of Nrf2 and therefore, its upregulation can enhance its nuclear transfer to induce antioxidant defense system.
There are several downstream factors regulated by Nrf2. In the regulation of antioxidant defense system and ROS levels, Nrf2 can affect expression level of HO-1, NQO1 and decrease the ROS levels to diminish oxidative damage. This balance and reinforcement of antioxidant defense system are required for the cell proliferation, as the high levels of ROS levels can impair the cellular and biological mechanisms [42]. In addition, Nrf2 is able to control the levels of cell cycle proteins including CDKs and cyclins to increase cell cycle progression and proliferation. Moreover, the CDK inhibitors including p21 and p27 are downregulated by Nrf2 [43]. The glucose metabolism can be increased by Nrf2 to provide the energy supplies for the biosynthesis and cell division in supporting cell proliferation [44]. Nrf2 also demonstrates interaction with oncogenes and onco-suppressor factors. As an example, p53 can suppress Nrf2 to decrease the cellular proliferation in response to stress signals [45]. PI3K/Akt and other related pathways are regulated by Nrf2 in controlling cell proliferation and survival. Nrf2 induces the phosphorylation of AKT in enhancing growth and viability [46]. Finally, the interaction of Nrf2 with the epigenetic factors can affect other genes in regulating proliferation.
The molecular profile of Nrf2 has been well-understood and the factors participating in its induction and the related proteins have been recognized. When there is an increase in reactive oxygen species (ROS), the protein Nrf2 is separated from Keap1 and translocates into the nucleus [47]. Keap1 is a member of the Kelch family of proteins (KLHL), and it attaches to the Neh2 domain of Nrf2 in a specific region covering amino acids 69 to 84 and adjacent to the ETGE motif [35]. This region has been located at the middle of protein. Keap1 is the protein accounting for the ubiquitination of the lysine in the Neh2 domain, leading to the degradation of Nrf2 by the proteasome. Keap1 provides the connection of Nrf2 with the ubiquitination ligase Cul-3 (also known as Cullin-3) [48]. Moreover, Nrf2 is affected by the different factors. These mechanisms include translational, post-translational, transcriptional, and epigenetic processes. The adaptor protein called p62 provides transporting ubiquitinated proteins to the autophagosome through Keap1 interaction, leading to the nuclear translocation of Nrf2 [49]. Noteworthy, Nrf2 utilizes p62 to regulate its own activity [50]. Additionally, P21 upregulates Nrf2 through direct interaction enhancing Nrf2 protein stability [51]. In adipocyte-associated macrophages, globular adiponectin (gAcrp) as a hormone can cause an increase in the generation of pro-inflammatory cytokines including tumour necrosis factor-alpha (TNF-α) and interleukin 1-beta (IL-1β). Moreover, it also enhances the levels of p62, reducing pro-inflammatory cytokines [52]. P62 also interacts with Keap1, increasing Keap1 degradation [49,53]. Nrf2 shows interaction with V-Maf Avian Musculoaponeurotic Fibrosarcoma Oncogene MAF proteins (sMAF) to generate heterodimers. Such heterodimers can control genes including antioxidant response elements (AREs) or MAF recognition elements (MAREs) [54,55]. Approximately 250 genes, the majority of which contribute to the endogenous antioxidant defence and the detoxification of ROS, are affected by the generation of Nrf2 [56,57]. The factors including NAD(P)H dehydrogenase (quinone) 1 (NQO1), Glutathione S-transferase 1 (GST) [58], heme oxygenase-1 (HMOX1), glutamate-cysteine ligase (GCL), peroxiredoxins, and enzymes associated to Glutathione (GSH) [59] synthesis are downstream targets of Nrf2. The mutations in Nrf2 or Keap1 can affect Nrf2 signaling [60]. The increasing evidence has shown that Nrf2 upregulation in cancer can accelerate progression and development drug resistance. Moreover, pharmacological compounds and natural products regulating Nrf2 in disease therapy have been introduced. The next sections emphasize on the function of Nrf2 in cancer progression and therapy resistance development.
Nrf2 in cancer proliferation and metastasis
Owing to the function of Nrf2 in cancer progression, the studies have focused on the role of this pathway in increasing proliferation and metastasis of tumor cells with an emphasis to the related pathways. Both proliferation and metastasis are the threats to the cancer patients, especially metastasis that allows the tumor cells for migrating into other parts of body and such dissemination leads to the establishment of new colonies for the cancer progression. The Nrf2 upregulation is not specific to a certain type of cancer and its upregulation can accelerate tumorigenesis in various cancer types. The presence of SPOP mutations in the prostate cancer can facilitate cancer progression. The SPOP mutations stimulate autophagy through p62/SQSTM1 axis and induce Nrf2 axis to promote cancer progression [61]. In the previous section, it was shown that p62 and Nrf2 have interactions and a continuation to the previous study could be understanding the interaction of p62 and Nrf2 after the SPOP mutations in prostate cancer. An interesting point is the co-regulation of Nrf2 and autophagy in the human cancers. The deficiency of PKCλ/ι can accelerate the progression of liver tumor and such loss of PKCλ/ι stimulates protective autophagy and enhances Nrf2 expression to facilitate progression of liver tumor [62]. The role of Nrf2 in the acceleration of cancer metastasis has been fully understood. The lung cancer metastasis is a threat to the human and the upregulation of Nrf2 prevents the degradation of Bach1 and through enhancing the stability of Bach1, Nrf2 enhances cancer invasion and migration [63]. The increase in the progression of cancer can endow the drug resistance in tumor cells. Although the role of Nrf2 in the stimulation of drug resistance is discussed with details in section 8, but it is of importance to mention that increase in the stability of Nrf2 can be induced by DUB3 to cause chemoresistance [64]. Since Nrf2 promotes proliferation and metastasis of cancer cells, the involvement of Nrf2 in the drug resistance is inevitable. Furthermore, the upregulation of Nrf2 can cause the poor prognosis in lung cancer [65]. Therefore, Nrf2 has the potential to be considered as a reliable and independent prognostic factor in cancer.
An important aspect is the involvement of Nrf2 in the metabolic reprogramming of cancer cells. The Nrf2 causes metabolic vulnerability in the lung cancer and through upregulation of ALDH3A1, Nrf2 stimulates NADH-reductive stress [66]. The function of Nrf2 in the regulation of metabolism in cancer is more evident by regulation of glycolysis. The glycolysis was first recognized in liver tumor [67] and it is characterized by increase in the uptake of glucose, lactate generation, implicating in enhancing proliferation, invasion, chemoresistance and immune evasion [68]. The progression of hepatocellular carcinoma can be suppressed by the function of UBR7. The UBR7 is upregulated by ALKBH5. Then, UBR7 increases Keap1 levels to suppress Nrf2/Bach1 axis, inhibiting HK2 function and suppressing glycolysis in disrupting the progression of hepatocellular carcinoma [69]. The stimulation of Nrf2 by upstream mediators can increase proliferation and metastasis. Therefore, silencing upstream inducers of Nrf2 can impair tumorigenesis. The knock-down of GLS1 can suppress Nrf2/autophagy axis to impair progression of colorectal cancer [70]. The increase in the progression of cancer can be obtained through the reducing cell death. The upregulation of APOC1 in glioblastoma suppresses Keap1 to upregulate Nrf2, preventing ferroptosis and improving the progression [71]. It appears that the tumor-promoting role of Nrf2 dependent on the De-glycation by fructosamine-3-kinase [72]. The increase in the metastasis of cancer cells can be obtained by the function of HIF-1α. The Nrf2 upregulation in breast cancer causes overexpression of G6PD that in turn, elevates levels of NADPH. The upregulated NADPH prevents ROS and through upregulation of HIF-1α, overexpression of Notch1 occurs to enhance metastasis of cancer cells through EMT induction [73]. The stabilization of Nrf2 by USP11 can increase progression and survival of tumor cells through inhibition of ferroptosis that is a good signature for ensuring tumorigenesis [74]. In order to suppress Nrf2 axis, the upregulation of Keap1 as the negative regulator of Nrf2 is suggested. MOAP-1 is able to cause dissociation of p62/SQSTM1 to cause Keap1 release for the suppression of Nrf2 [75]. Hence, these studies briefly demonstrate role of Nrf2 in increasing proliferation and metastasis, summarized in Table 1 and Figure 2.
Table 1.
The function of Nrf2 in increasing cancer progression
| Cancer | Molecular profile | Remark | Ref |
|---|---|---|---|
| Thyorid cancer | TIAM1/Nrf2/HO-1 | ALKBH5 suppresses Nrf2/HO-1 axis through regulating TIAM1 to induce ferroptosis and suppress cancer progression | [76] |
| Pancreatic cancer | TRIM2/Nrf2 | TRIM2 promotes stimulates ROS/Nrf2 axis to enhance FAK levels in tumorigenesis | [77] |
| Lung cancer | Nestin/Keap1/Nrf2 | Nestin competes with Keap1 to increase stability of Nrf2 | [78] |
| Colon cancer | ARD1 | ARD1 increases stability of Nrf2 in colon tumorigenesis | [79] |
| Bladder cancer | WTAP | WTAP increases Nrf2 stability to prevent ferroptosis | [80] |
| Cervical cancer | UCHL3 | UCHL3 increases Nrf2 stability to cause metastasis | [81] |
| Gastric cancer | GSTM3 | GSTM3 is upregulated by Nrf2 | [82] |
| Due to presence of positive feedback loop, GSTM3 competes with Keap1 to increase Nrf2 stability | |||
| Colon cancer | Nrf2 | Nrf2 controls phosphorylation of Akt and ERK in the regulation of apoptosis and cancer progression | [83] |
| Prostate cancer | Nrf2 | Nrf2 silencing increases cell death and sensitizes to cisplatin | [84] |
| Lung cancer | Nrf2 | For exerting the proliferative function of Nrf2, the presence of ERBB3 and IGF1R is required | [85] |
| Osteosarcoma | DDRGK1 | DDRGK1 promotes Nrf2 expression through Keap1 suppression to accelerate progression and induce drug resistance | [86] |
| Prostate cancer | STC1 | STC1 depletion suppresses Nrf2/HO-1 axis to impair glycolysis and induce ferroptosis | [87] |
| Prostate cancer | - | A combination of Nrf2 inhibitor and autophagy suppressor synergistically suppress tumor progression | [88] |
| Lung cancer | BRD4 | BRD4 suppresses Nrf2 activity to disrupt redox metabolism | [89] |
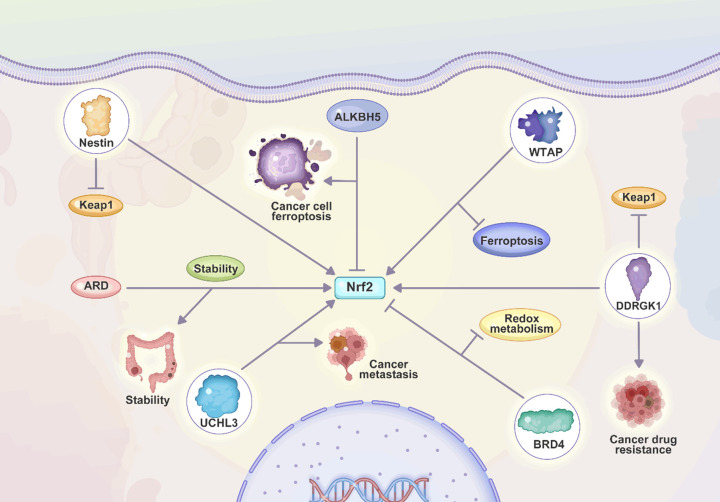
Figure 2.
Association of Nrf2 with the proliferation and metastasis of tumor cells. The upregulation of Nrf2 by UCHL3 can enhance the metastasis of cancer cells. Moreover, nestin impairs Keap1 to upregulate Nrf2 in causing tumorigenesis. The upregulation of Nrf2 by WTAP reduces ferroptosis, while ALKBH5 promotes ferroptosis through Nrf2 downregulation. Moreover, DDRGK1 induces drug resistance through Nrf2 upregulation.
Nrf2 and cell death mechanisms
Ferroptosis
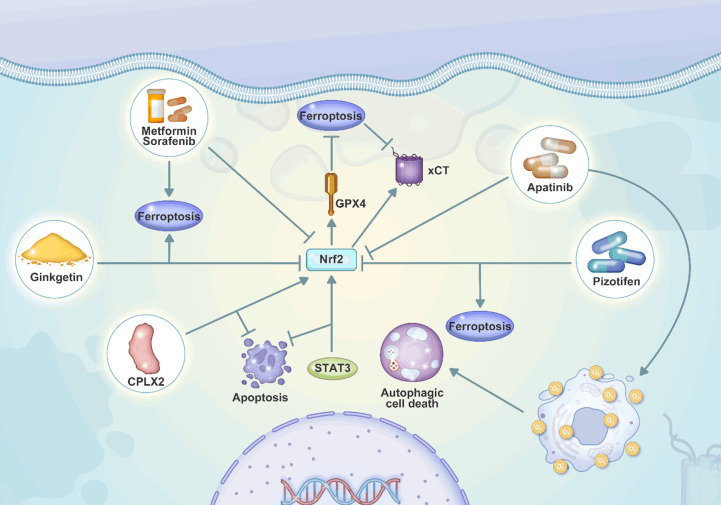
Iron accumulation and lipid peroxidation are the two hallmarks of ferroptosis, as a kind of cell death. In contrast to other well-known processes of cell death including apoptosis, necroptosis, and autophagy, ferroptosis has received much attention [90]. By inhibiting the generation of lipid peroxides, the GPX4 is a potent regulator of ferroptosis [91] that its upregulation prevents ferroptosis. The xCT antiporter system is another essential component that is linked to the mechanism of ferroptosis. This system is essential for the transfer of extracellular cystine for intracellular glutamate, which is a precursor for the antioxidant glutathione (GSH) [92]. Inhibiting xCT and GPX4 can result in the death of cancer cells that have developed resistance to conventional chemotherapy and radiation treatment [93]. Blocking xCT reduces the amount of GSH in the body through suppressing the uptake of cysteine, improving drug sensitivity [94,95]. On the other hand, mesenchymal cancer cells that are resistant to therapy can be rendered sensitive to ferroptotic cell death by blocking GPX4 [96]. Targeting the lipid peroxidation pathway that protects against ferroptosis can make therapy-resistant cancer cells more susceptible to the ferroptosis-induced form of cell death [97]. Since ferroptosis can significantly improve suppressing cancer progression, the studies have focused on application of GPX4 inhibitors to accelerate ferroptosis. However, the upregulation of Nrf2 can cause resistance to these compounds. As a result, downregulation of Nrf2 can increase the sensitivity to GPX4 inhibitor-mediated ferroptosis in head and neck cancer [98]. This is also repeated for the colorectal cancer and the administration of cetuximab can inhibit Nrf2/HO-1 axis to promote ferroptosis [99]. Therefore, Nrf2 upregulation is considered as a barrier towards the stimulation of ferroptosis in cancer. The increase in the expression of Nrf2 promotes xCT levels to prevent ferroptosis, accelerating the proliferation of cancer [100]. When the ferroptosis is suppressed in cancer, it can cause the development of chemoresistance. The downregulation of Nrf2 increases ferroptosis and prevents cisplatin resistance in head and neck cancer [101]. The upregulation of Nrf2 in cancer can promote GPX4 that is an impediment towards ferroptosis induction. The overexpression of Nrf2/GPX4 axis is mediated by KIF20A through enhancing NUAK1 expression to cause ferroptosis inhibition and enhance oxaliplatin resistance in colorectal cancer [102]. Due to the function of LTBP2 in increasing Nrf2 expression, it upregulates GPX4 and xCT to induce resistance to ferroptosis in gastric tumor [103]. The application of new genetic tools such as CRISPR has enabled to screen the factors regulating ferroptosis in cancer that upregulation of Nrf2 and its association with GPX4 can prevent ferroptosis [104]. The increase in the degradation of Nrf2 by MIB1 can increase the ferroptosis induction in cancer [103]. Therefore, the upregulation of Nrf2 suppresses ferroptosis mainly through induction of antioxidant defense system and overexpression of GPX4. Table 2 summarizes Nrf2 and ferroptosis interaction in cancer.
Table 2.
The Nrf2 and ferroptosis interaction in cancer
| Molecular profile | Remark | Ref |
|---|---|---|
| HIF-1α/IRP1/TFR1 and Keap1/Nrf2/GPX4 | Trabectedin stimulates ferroptosis in lung cancer through suppressing Nrf2/GPX4 and upregulation of TFR1 | [105] |
| Nrf2/HO-1 | Nrf2/HO-1 suppression by ginkgetin to stimulate ferroptosis in lung cancer | [106] |
| AMPK/Nrf2/HMOX1 | AMPK/Nrf2/HMOX1 stimulation by vitamin C to accelerate ferroptosis in pancreatic cancer | [107] |
| P62/Keap1/Nrf2 | Metformin and sorafenib combination stimulates ferroptosis in hepatocellular carcinoma through Nrf2 downregulation | [108] |
| Keap1/Nrf2/HO-1 | Ferroptosis induction and stimulation of Keap1/Nrf2 suppress EMT in gastric cancer | [109] |
| P62/Keap1/Nrf2 | L-selenocystine’s ability to inhibit Nrf2 and autophagy, along with its effect on the p62-Keap-1-Nrf2 axis, leads to the selective death of Nrf2-dependent colorectal cancer cells | [110] |
| Nrf2 | The efficacy of phototherapy in tumor suppression increases by Nrf2 suppression and ferroptosis induction | [111] |
| GSK3β/Nrf2 | Silenced GSK3β increases Nrf2 expression to suppress ferroptosis in breast cancer | [112] |
| miR-365a-3p/NRF2 | MT1DP loaded by folate-modified liposomes makes non-small cell lung cancer cells more sensitive to the effects of erastin-induced ferroptosis via modulating the miR-365a-3p/NRF2 axis | [113] |
| CAMKK2 | CAMKK2 increases Nrf2 expression to suppress ferroptosis in melanoma | [114] |
| IGF2BP3/Nrf2 | IGF2BP3 increases Nrf2 stability to suppress ferroptosis in hepatocellular carcinoma | [115] |
| Nrf2 | Impairing the function of Nrf2 can increase ferroptosis in hepatocellular carcinoma | [116] |
| Nrf2/xCT | AdipoR1 suppresses Nrf2/xCT axis to enhance ferroptosis in hepatocellular carcinoma | [117] |
| VEGFR2/Nrf2 | Apatinib suppresses VEGFR2/Nrf2 to induce ferroptosis in glioma | [118] |
| Nrf2 | Pizotifen suppresses Nrf2 to increase ferroptosis in esophageal cancer | [119] |
| Nrf2/HO-1 | Nrf2/HO-1 downregulation by metformin in ferroptosis induction | [120] |
| Nrf2 | Oxaliplatin downregulates Nrf2 to increase oxidative damage and ferroptosis | [121] |
| Nrf2 | Increased degradation of Nrf2 by lysionotin in ferroptosis induction | [122] |
| Nrf2/GPX4 | Nrf2/GPX4 suppression by wogonin to induce ferroptosis in pancreatic cancer | [123] |
| Nrf2 | Nrf2 downregulation by ibrutinib to mediate ferroptosis in colorectal cancer | [124] |
| NRF2/HO-1 | NRF2/HO-1 downregulation by norcantharidin to stimulate ferroptosis in ovarian cancer | [125] |
| Nrf2/HO-1 | Nrf2/HO-1 stimulation by levistilide a to mediate fgerroptosis in breast cancer | [126] |
| Nrf2/GPX4 | AGuIX nanostructures suppress Nrf2/GPX4 axis to increase ferroptosis in cancer | [127] |
| Stat3/p53/NRF2 | Ginsenoside Rh3 downregulates Nrf2 to accelerate ferroptosis in colorectal cancer | [128] |
| Nrf2/HO-1/NQO1 | Eriodictyol suppresses Nrf2/HO-1/NQO1 to mediate ferroptosis and mitochondrial dysfunction in ovarian cancer | [129] |
| SIRT6/Nrf2/GPX4 | Isoorientin suppresses SIRT6/Nrf2/GPX4 axis to enhance ferroptosis in lung cancer | [130] |
| Nrf2/HO-1 | Acetaminophen suppresses Nrf2/HO-1 axis to induce ferroptosis in lung cancer | [131] |
Apoptosis
There is a requirement for providing the balance between cell survival and death [132]; nevertheless, disruption in this balance can lead to the development of diseases including cancer. Apoptosis, categorized into extrinsic or the intrinsic pathways, is one of the most important forms of programmed cell death, which was first detected in the 1840s during the development of toads [133]. This was the first observation of cell death. A kind of protease called caspase is essential for the morphological and biochemical alterations occurring in apoptotic cells. These alterations are caused by the caspases [134,135]. The extrinsic route participates in the binding of death receptors to their respective ligands, which subsequently activates caspases, which in turn triggers apoptosis [135]. An intricate interaction occurring between pro-apoptotic and anti-apoptotic BCL-2 family proteins throughout the intrinsic route, known as mitochondrial apoptosis [136]. The interaction of Nrf2 and apoptosis can determine the progression of cancer. The regulation of apoptosis and ferroptosis in hepatocellular carcinoma can be provided by CPLX2; the downregulation of NRF2 occurs as a result of CPLX2 depletion, showing the role of this factor in inhibition of apoptosis and ferroptosis [137]. However, there is a controversy that phloretin can stimulate mitochondrial apoptosis through STAT3 downregulation and subsequent Nrf2 upregulation to impair progression, proliferation and invasion of pancreatic cancer [138]. Noteworthy, the suppression of protective autophagy and Nrf2 downregulation by fisetin can accelerate apoptosis in colorectal cancer [139]. Therefore, Nrf2 has ability of regulating apoptosis in human cancers and its function is dual and like a double-edged sword.
Autophagy
Autophagy is considered as one of the highly conserved evolutionary mechanisms. The degradation of different cellular components including cytoplasmic macromolecules, aggregated proteins, damaged organelles, or infections, is a part of this process. Lysosomal enzymes are accountable for the degradation of these components upon their transfer to lysosomes. As a result of this degradation, nucleotides, amino acids, fatty acids, sugars, and ATP are recycled, all of which are subsequently recycled back into the cytoplasm of the cell. Overall, autophagy functions as a double-edged sword. Autophagy is vital for the elimination of damaged proteins and organelles, thereby conserving the quality and quantity of cellular components. Moreover, it participates in preserving the metabolism during stress signals [140]. The initiation of autophagy occurs through the generation of isolated membranes known as phagophores. The origin of these membranes is believed to be the endoplasmic reticulum, trans-Golgi, and endosomes. This phagophore engulfs the intracellular components including protein aggregates, organelles, and ribosomes, leading to the formation of a double-membrane structure called autophagosome. Then, autophagosomes fuses with lysosomes upon maturation to degrade the content using proteases. Using this mechanism, lysosomal transporters participate in the transfer of amino acids and other by-products of degradation back into the cytoplasm. After recycling, these components can re-use for the synthesis of macromolecules and preserving cellular metabolism [141]. The increasing evidence has shown the potential of autophagy in the regulation of cancer progression, drug resistance and as a therapeutic target of pharmacological compounds [142,143]. The experiments have highlighted the interaction of autophagy and Nrf2 in the tumor progression. The upregulation of p62 can suppress autophagy. Apatinib increases ROS levels to suppress Nrf2 axis, leading to p62 downregulation and increase in autophagic cell death [144]. The overexpression of BDH2 can increases Nrf2 degradation through the function of Keap1. Then, Nrf2 function is suppressed to increase ROS levels in impairing the Akt and mTOR phosphorylation, causing autophagy in gastric cancer therapy [113]. The upregulation of TRIM13 in lung cancer can increase Nrf2 suppression and through p62 degradation, it stimulates autophagy and disrupts the cancer progression [145]. However, a complexity at the present is the dual function of autophagy. Although the previous studies highlighted the role of autophagy and its interaction with Nrf2 in tumor suppression, the stimulation of autophagy can also accelerate tumorigenesis, known as protective or carcinogenic autophagy. The overexpression of Nrf2 in lung cancer can stimulate autophagy in facilitating tumor progression [146]. Moreover, the exposure of cancer cells to the treatment should be performed carefully due to the interaction of Nrf2 with autophagy mechanism. The treatment of lung cancer with isodeoxyelephantopin increases nuclear transfer of Nrf2 due to the Keap1 degradation. Then, Nrf2 promotes HO-1 and p62 levels. The overexpression p62 further degrades Keap1 to induce Nrf2 axis and on the other hand, p62 promotes survival of lung tumor through autophagy induction [147]. The interaction of Nrf2 and p62 has been a problem in the treatment of cancer. The overexpression of JNK/ERK in cancer can stimulate autophagy through Beclin-1 upregulation, causing autophagy and increasing p62 levels. Then, p62 suppresses Keap1 to stimulate Nrf2 axis, enhancing proliferation of cancer [148]. Since autophagy can also accelerate cancer progression, the dual suppression of autophagy and Nrf2 can stimulate apoptosis through increasing ROS generation and causing endoplasmic reticulum (ER) stress in pancreatic cancer [149]. Therefore, the accumulating data demonstrate the interaction of Nrf2 and autophagy in cancer [150-154].
Pyroptosis
A kind of cell death was identified in 1992 in mouse macrophages that had been infected with Shigella flexneri [155]. This cell death was a result of the infection. The induction of a protein called inflammatory caspase-1 occurred during the process of cell death that was caused by Shigella flexneri or Salmonella [156,157]. At first, it was believed that this kind of cell death was a caspase-dependent process mimicking apoptosis. On the other hand, Cookson and colleagues made the discovery in 2001 that the death of cells caused by Salmonella had specific properties that were unique from apoptosis. This kind of cell death was called pyroptosis, since in contrast to apoptotic cells, macrophages infected with Salmonella undergo cell enlargement and membrane degradation [158,159]. This led to the using word “pyroptosis” to characterize it from apoptosis [159]. The development of pores in the cell membrane, the rupture of the membrane, the enlargement of the cell, and the discharge of the contents of the cell are all hallmarks of the pyroptotic. IL-1 and IL-18 are released during pyroptosis and have the dual function of both increasing inflammation and stimulating immunological responses [160,161]. The interaction of Nrf2 and pyroptosis is observed in human cancers. The overexpression of Nrf2 can suppress both ferroptosis and pyroptosis. Nrf2 reduces ROS levels to downregulate caspase-3. The reduced caspase-3 downregulates GSDME-N to prevent pyroptosis. Moreover, Nrf2 promotes GPX4 levels to suppress ferroptosis [162].
Necroptosis
Necroptosis is a kind of inflammatory cell death that is observed upon the upregulation of death domain receptors. Necroptosis was first found as an alternative to apoptosis. This kind of cell death, distinct from apoptosis, is related to infections and wound healing. The classical death receptor-mediated necroptotic pathway comprised of RIPK1-RIPK3-MLKL, is induced upon the stimulation of death domain receptors including TNFR and Fas, and Toll-like receptor-3/4 (TLR3/4) [163]. The microliposomes containing B2 plasmid can accelerate Nrf2-regulated oxidative damage to increase necroptosis through upregulation of RIPK3 (Figure 3) [164].
Figure 3.
Nrf2 and cell death mechanisms in cancer. The main regulation is related to the apoptosis, autophagy and ferroptosis. About the regulation of autophagy by Nrf2, it should be considered that function of autophagy in cancer is like a double-edged sword and it has ability of impairing/accelerating tumorigenesis and reversing/inducing drug resistance. Moreover, upregulation of autophagy can prevent apoptosis and ferroptosis in tumor cells.
Nrf2 mechanism and EMT
Most of the cancer-related deaths are due to the metastasis and therefore, it is of high importance to understand the mechanisms involved in the metastasis regulation. Jean Claude first explored the phrase “metastasis” in 1829. It comes from the Greek terms “meta”, meaning “next”, and “stasis”, meaning “placement”. Metastasis refers to the spread and invasion of cancerous cells from the primary site to other areas of the body, which can ultimately result in the development of secondary tumours and colonies and is one of the main reasons of cancer-related death. EMT is a kind of biological mechanism contributing to cancer metastasis. Recent studies have shown that EMT plays a critical part in cancer cell invasion and therapy resistance. During EMT, cells experience loss of adhesion between neighbouring cells and the extracellular matrix. This leads to the cells features that are more typical of mesenchymal cells. This leads to the detachment of cells from the original colony and infiltration into the tissues and organs in the surrounding area and distant regions. In addition to cancer, EMT also plays an essential role in the development of embryos, the formation of scar tissue and wound healing [165,166]. The stimulation of EMT has been considered as a factor in enhancing tumor progression, metastasis, dissemination and chemoresistance [167,168]. The stimulation of Nrf2 can promote metastasis of cancer through EMT induction. The presence of lactic acid can increase Nrf2 levels to induce M2 polarization of macrophages. Then, they secrete VEGF to increase Nrf2 transfer to nucleus in EMT induction [169]. Since Nrf2 is related to ROS and ROS levels are changed by mitochondria, there is an interaction between mitochondria and Nrf2. The increased levels of Ca2+ levels in the cytoplasm can transfer into mitochondria to activate MCUR1 in increasing ROS levels. The upregulation of ROS to promote Nrf2 levels to upregulate NICD1. Then, NICD1 increases Snail expression to induce EMT in hepatocellular carcinoma invasion [170]. Noteworthy, the function of ROS is more than regulating Nrf2 and it can bind to promoter of genes. The ROS can induce hypomethylation of PRDX5 in upregulation of STAT3, inducing Nrf2 axis and promoting EMT in lung cancer invasion [171]. On the other hand, since STAT3 is a cytokine-related factor, the high levels of IL-6 can induce STAT3 to promote Nrf2 levels in causing EMT in pancreatic cancer [172]. The upregulation of UBE2E2 in the nucleus promotes Nrf2 expression. Then, levels of p62 increase to overexpress Snail in EMT induction through E-cadherin downregulation [173]. The therapeutic compounds regulating Nrf2 can impair metastasis of cancer cells. Pedunculoside downregulates Nrf2 and MAPK to suppress EMT, metastasis and gefitinib resistance [174]. Jolly and colleagues have provided a hypothesis that Nrf2 upregulation causes partial EMT and can cause hybrid EMT phenotype [175]. The presence of hypoxia can also increase the progression of cancer. Hypoxia increases Nrf2 expression to downregulate miR-27a, inducing EMT and enhancing cancer progression [176]. Moreover, the overexpression of Nrf2 can increase Notch1 expression to induce EMT through N-cadherin overexpression and E-cadherin downregulation [177].
Non-coding RNAs in the regulation of Nrf2
The non-coding RNAs are a large family of RNAs lacking protein coding potential and they contribute to the major functions including biological and molecular pathway control. The first type is microRNAs (miRNAs) with short length less than 25 nts and capacity of gene expression modulation or translation suppressing. The recent studies have highlighted the mutual interaction of miRNAs and Nrf2 in human tumors. In the cancer cells showing Nrf2 downregulation, there is upregulation of miR-206 that can downregulate c-MET and EGFR [178]. Therefore, it can be concluded that miRNA expression can be also regulated by Nrf2. The regulation of Nrf2 by miRNAs can change the process of cancer progression. miR-141-3p upregulation in ovarian tumor accelerates tumorigenesis and it stimulates the M2 polarization of macrophages through Keap1 suppression and subsequent induction of Nrf2 [179]. However, when miRNAs suppress Nrf2 axis, the progression of cancer reduces. The breast tumor progression significantly enhances in hypoxic condition. miR-140-5p downregulates Nrf2/HO-1 axis to impair progression in hypoxia [180]. The miRNAs can directly and indirectly regulate the expression level of Nrf2. SIRT1 stimulates Nrf2 in increasing progression of pancreatic cancer. miR-373 downregulates SIRT1 expression to suppress Nrf2 axis, increases BAX and caspase expression and stimulate oxidative damage, impairing tumor proliferation and triggering apoptosis [181]. The interesting point is that miRNA expression can be increased by Nrf2 in enhancing tumor progression. Nrf2 is able to enhance levels of miR-125B, while it downregulates miR-29B to accelerate progression of leukemia and increase survival of cancer cells [182]. Furthermore, the upregulation of Nrf2 by miR-155 can prevent apoptosis, causing arsenic trioxide resistance in lung tumor [183]. However, miRNAs are not the only regulators of Nrf2 in cancer and circular RNAs (circRNAs) are another type that can participate in carcinogenic modulation. CircRNAs, have recently been recognised as important players in the field of cancer biology. These non-coding RNA molecules are distinguished by the presence of a loop structure that is covalently closed. This structure gives resistance to the cleavage of the loop by exonucleases. CircRNAs have a role in a variety of characteristics of cancer, including carcinogenesis, treatment resistance, and metastasis. They perform this by a variety of methods, including as sponging miRNAs, interacting with RNA-binding proteins, and influencing alternative splicing. These processes all coordinate together to modulate gene expression. Depending on their targets and the environment within the cell, circular RNAs have the potential to either promote or inhibit the growth of tumours. Because the dysregulation of these circRNAs is linked to a wide variety of malignancies, they provide intriguing diagnostic and therapeutic targets for the treatment and management of cancer. The function of circRNAs in the cancer can be found in some recent reviews [184-186]. The circKEAP1 is able to impair progression of lung cancer. CircRNAs have ability of sponging miRNAs in the regulation of Nrf2. CircKEAP1 sponges miR-141-3p and through upregulation of Keap1, it impairs Nrf2 axis to reduce progression of lung cancer [187]. Another member of non-coding RNA family is long non-coding RNAs (lncRNAs). Long non-coding RNAs, often known as lncRNAs, have recently been recognised as important players in the field of cancer biology. These non-protein-coding RNA molecules, which are generally longer than 200 nts, are implicated in a variety of the processes that lead to the development of tumours. LncRNAs are capable of influencing gene expression, cell proliferation, and metastasis, and can function either as oncogenes or tumour suppressors. LncRNAs are also involved in the pathways, leading to the therapy resistance, and they have the potential to act as biomarkers for the diagnosis and prognosis of cancer. When it comes to cancer, having a comprehensive understanding of the complex functions that lncRNAs play opens up new gateways for the development of therapeutics and personalised treatment regimens. The function of lncRNAs in cancer can be found in some recent reviews [188-190]. The lncRNA-mediated regulation of Nrf2 can also determine the fate and progressive behaviour of cancer. Moreover, the lncRNAs and circRNAs have similarity in the standpoint that both of them can sponge miRNAs and regulate their expression. The low expression of lncRNA SLC7A11-AS1 can impair tumor progression. The upregulation of lncRNA SLC7A11-AS1 promotes the levels of SLC7A11 and enhances the nuclear transfer of Nrf2, causing tumorigenesis in colorectal cancer [191]. Furthermore, the interaction of lncRNAs and Nrf2 can determine the chemotherapy resistance in cancer. The lncRNA MIR4435-2HG upregulates Nrf2 and HO-1 expression to trigger cisplatin resistance (Table 3) [192]. Table 3 summarizes the non-coding RNA and Nrf2 interaction in cancer. Figure 4 shows the regulation of Nrf2 by non-coding RNAs.
Table 3.
The regulation of Nrf2 by non-coding RNAs in cancer
| Cancer type | Non-coding RNA | Remark | Ref |
|---|---|---|---|
| Breast cancer | miR-200a | miR-200a increases Nrf2 expression through Keap1 downregulation to induce antioxidant signalling | [193] |
| Breast cancer | miR-101 | Nrf2 downregulation by miR-101 to induce apoptosis | [194] |
| Lung cancer | miR-144-3p | Nrf2 suppresses activation of miR-144-3p to induce cisplatin resistance | [195] |
| Esophageal cancer | miR-142-5p | Upregulation of miR-142-5p by polygalacin D downregulates Nrf2 in cancer suppression and apoptosis induction | [196] |
| Lung cancer | miR-1290 | Upregulation of miR-1290 by COX-2 stimulates in CAF induction and enhancing cancer progression | [197] |
| Esophageal cancer | miR-27b-3p | miR-27b-3p downregulates Nrf2 to suppress cancer progression | [198] |
| Breast cancer | miR-93 | miR-93 suppresses Nrf2 expression | [199] |
| Hepatocellular carcinoma | miR-340-5p | NRAL stimulates cisplatin resistance through miR-340-5p sponging to upregulate Nrf2 | [200] |
| Hepatocellular carcinoma | miR-101 | Apigenin increases miR-101 expression to suppress Nrf2 axis | [201] |
| Hepatocellular carcinoma | miR-340 | miR-340 downregulates Nrf2 in suppressing cisplatin resistance | [202] |
| Hepatocellular carcinoma | miR-141 | miR-141 stimulates Nrf2 through Keap1 suppression in drug resistance | [203] |
| Leukemia | miR-144-3p | miR-144-3p downregulates Nrf2 expression | [204] |
| Prostate cancer | LMNTD2-AS1 | LMNTD2-AS1 interacts with Nrf2 through binding to FUS in increasing cancer progression | [205] |
| Prostate cancer | TUG1 | TUG1 increases Nrf2 expression to elevate proliferation and invasion | [206] |
| Esophageal cancer | TUG1 | TUG1 upregulates Nrf2 to induce cisplatin resistance | [207] |
| Colorectal cancer | LINC00239 | LINC00239 increases Nrf2 stability through Keap1 inhibition for suppressing ferroptosis | [208] |
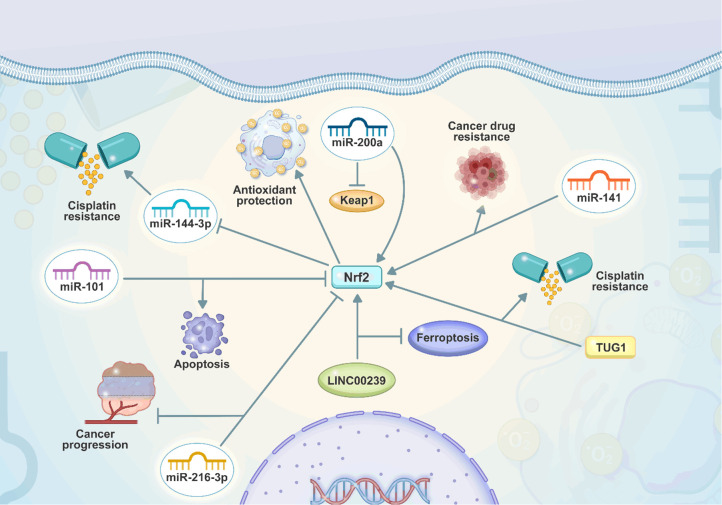
Figure 4.
The regulation of Nrf2 by non-coding RNAs in cancer. The miRNAs can change the expression of Nrf2 by binding to 3’-UTR. Moreover, the Nrf2 downregulates miR-144-3p expression to increase cisplatin resistance. miR-101 induces apoptosis through Nrf2 downregulation. Moreover, LINC00239 impairs ferroptosis through upregulation of Nrf2.
Nrf2 in resistance
One of the biggest hurdles for the treatment of cancer is chemoresistance. The different mechanisms participating in the development of resistance have been identified. These processes occur due to the heterogeneity, oncogenic mutations, epigenetic changes, cancer stem cell, and interactions with the surrounding environment, including the immune system and the microenvironment. Moreover, these mechanisms include interactions between the tumour and cancer stem cells. The anti-cancer impacts caused by conventional chemotherapy are based of increasing ROS levels. However, tumor cells have shown potential in adapting into this condition, causing drug resistance. This can happen whether the oxidative stress is caused spontaneously or by pharmaceuticals [209]. The word drug resistance is considered as the capacity of cancer cells to survive even upon exposure to various anti-cancer compounds [210]. The researchers have focused on understanding the underlying molecular pathways participating in the drug resistance. This has been evaluated from the different standpoints [211,212]. According to Bukowski’s research in 2020 [212], drug resistance can be intrinsic or acquired and can resulting from certain genetic and epigenetic changes as well as alterations in the tumour microenvironment components and interactions. The drug resistance also results from the genetic mutations. The acidic pH of tumor microenvironment also determines the drug resistance. Taylor in 2015 and Andrei in 2020 [213,214] brought attention to the development of unique 3D systems mimicking the tumor microenvironment as well as proton pump inhibitors (PPIs) as complicated tools for the purpose of better understanding drug resistance in cancer and finding ways to overcome it. According to a research [215], the alterations in the cellular processes can result in drug resistance. A number of these mechanisms include oxidative stress, DNA damage repair, apoptosis, and autophagy. Furthermore, increase in drug efflux, organelle sequestration, metabolism, and targeting contribute to the chemoresistance [216].
The increasing evidence has shown the role of Nrf2 in the stimulation of drug and cell death resistance in human cancers. Since genetic mutations and dysregulation of molecular pathways commonly occur in cancer, understanding the role of Nrf2 in tumorigenesis and drug resistance can broaden the knowledge towards the development more effective therapeutics in the near future. FAM117B is capable of inducing chemoresistance in gastric cancer through Keap1 suppression to upregulate Nrf2 [119]. Therefore, suppression of Nrf2 can impair the process of chemoresistance. The administration of tangerein can suppress Nrf2 axis in suppressing tumor proliferation and drug resistance [217]. This study highlights the function of Nrf2 in chemoresistance and evaluating it as a “druggable target” to reverse this condition. One of the leading causes of death around the world is esophageal cancer in which 5-year survival rate of this tumor is lower than 20%. Chemotherapy and radiation are the common therapeutic modalities for esophageal cancer. However, the dysregulation of molecular factors in the esophageal cancer can cause chemoresistance. The downregulation of SOX17 and subsequent overexpression of Nrf2 cause drug resistance in esophageal cancer [121]. In the normal conditions, the ubiquitination of Nrf2 by Keap1/Cul3 complex occurs to induce Nrf2 degradation. However, during cisplatin resistance development, the function of Keap1/Cul3 is disturbed. Then, upregulation of Nrf2 occurs that upon epigenetic modification of target genes including GPX4, GCLC, GCLM, HO-1 and GSH, among others, the apoptosis and ferroptosis are suppressed to enhance growth and progression of cancer cells in drug resistance development [218]. For ubiquitination of Nrf2, the K48-linked polyubiquitin chains are affected that USP8 prevents the ubiquitination of such chains in the structure of Nrf2 in improving its stability and inducing gemcitabine resistance in pancreatic cancer [219]. The expression level of Nrf2 can also be regulated by the hormones and therefore, the development of drug resistance is more evident in the tumor cells that are dependent on the hormones for their progression. In the prostate cancer, the presence of hormones can accelerate the process of cancer drug resistance development. The estrogen and estradiol (E2) can enter the cancer cells and transfer the nucleus. Then, ERα transfers to nucleus and comprises a complex, called E2/ERα complex that interacts with ERE to upregulate Nrf2. The activated Nrf2 axis increases levels of GCLC, ABCC2, ABCB1, ABCG2, Bcl-2, CD44 and others to reduce ROS levels, causing chemoresistance in prostate cancer [220]. Therefore, the reason for the cancer drug resistance development is mainly due to the function of Nrf2 in increasing the expression level and activity of downstream targets that support tumor cells against insults. The upregulation of Nrf2 can occur through TMED2 function in inhibiting Keap1, causing upregulation of HO-1 and NQO1 to induce cisplatin resistance in breast cancer [221]. It appears that Nrf2 participates in the intrinsic resistance. For the cisplatin, it should increase the levels of ROS in activation of JNK, upregulation of caspase-3 and -9 to induce apoptosis. Therefore, increase in the levels of ROS is vital for apoptosis induction in tumor cells by cisplatin. However, upregulation of Nrf2 through TNFAIP2 can overexpress HMOX1, NQO1, SOD2 and CAT in reducing ROS levels and inducing cisplatin resistance [222]. The function of Nrf2 in causing drug resistance has been confirmed in different human cancers including renal cancer [223], lung carcinoma [224-226], breast cancer [227], bladder tumor [228], ovarian cancer [229] and leukemia [230], conferring the versatile function of Nrf2 in cancer drug resistance.
Natural products and pharmacological compounds targeting Nrf2
The function of Nrf2 in the regulation of carcinogenesis has been well-documented. Noteworthy, since Nrf2 is a “druggable target”, it can be affected by natural and pharmacological compounds in the treatment of cancer. Although the previous sections demonstrate the oncogenic function of Nrf2 and it creates the notion that Nrf2 should be suppressed, the anti-cancer compounds stimulate/suppress Nrf2 in the treatment of cancer. One of the issues is the role of Nrf2 in the stimulation of ferroptosis resistance. It has been reported that tagitinin C is able to stimulate ER stress to promote Nrf2 expression, upregulating HO-1, increasing lipid peroxidation and causing ferroptosis in colorectal cancer [231]. This study highlights the fact that stimulation of Nrf2 can enhance the lipid peroxidation to cause ferroptosis in tumor cells, while previous studies have shown the role of Nrf2 upregulation in stimulation of ferroptosis resistance. Therefore, the function of Nrf2 requires more investigation in cancer and maybe it shows double function in cancer. 4,4’-dimethoxychalcone (DMC) has capacity of suppressing cancer growth and stimulation of G2/m arrest. DMC promotes Keap1 degradation through ubiquitin pathway to induce Nrf2/HMOX1 axis. Moreover, DMC suppresses FECH to accelerate ferroptosis in cancer [232]. Considering the previous studies, a new hypothesis reveals that neferine has ability of ferroptosis induction in thyroid cancer. Neferine suppresses Nrf2 axis to downregulate HO-1 and NQO1 in stimulation of ferroptosis in thyroid tumor [233]. Therefore, function of Nrf2 in cancer is like a double-edged sword and its induction or stimulation can impair cancer progression. Noteworthy, when Nrf2 expression is regulated, it can affect the drug sensitivity and stemness of cancer cells. The luteolin administration can downregulate Nrf2, HO-1 and Cripto-1 to impair stemness and increase drug sensitivity in breast cancer [234]. For the treatment of endometrial hyperplasia and cancer, the application of progestin is suggested. However, the tumor cells have ability of developing resistance to these therapies. Brusatol suppresses NRF2/TET1/AKR1C1 to enhance the response of cancer cells to progestin [235]. However, if expression level of Nrf2 increases in response to a drug, it can cause cancer progression. The propofol administration promotes Nrf2 expression to impair ferroptosis and enhance distant invasion of cancer [236]. Therefore, it is suggested to use the compounds suppressing Nrf2 for the treatment of cancer.
Nanoparticles as Nrf2 inhibitors
The field of nanotechnology has received much attention in the recent years for the treatment of cancer. The nanoparticles can provide the sustained delivery of drugs [237] and they can be used for targeting specific mechanisms for the progression suppression of cancer cells [238-240]. In the current section, the role of nanostructures for the treatment of cancer through regulation of Nrf2 is discussed. The best strategy is to load siRNA suppressing Nrf2 in nanoparticles to specifically deliver into cancer cells. The nanobubbles are able to deliver Nrf2-siRNA and they release it in response to ultrasound to enhance the response of melanoma cells to cisplatin therapy. The encapsulation efficiency of these nanobubbles is 90% and they demonstrate high cellular uptake [241]. Another strategy is to load chemotherapy drugs in nanoparticles for increasing their potential. In the recent years, the process of loading chemotherapy drugs in nanostructures for suppressing cancer progression has significantly improved tumor elimination activity [242]. The PLGA-PEG nanoparticles can deliver oridonin to breast cancer cells, suppressing Nrf2 axis in increasing ROS production and causing apoptosis [243]. However, an experiment highlights the fact that stimulation of Nrf2 and ROS generation by β-Sitosterol-assisted silver nanostructures can trigger mitochondrial apoptosis in hepatocellular carcinoma [244]. The nanoparticles can affect the underlying mechanisms regulating Nrf2 in cancer progression regulation. The zero-valent-iron nanostructures enhance the AMPK levels, while they suppress mTOR to upregulate GSK3β and β-TrCP. Moreover, this results in downregulation of Nrf2 to reduce levels of SLC7A11, GPX4, AIFM2 and AKR1 in causing ferroptosis and impairing progression of cancer [245]. Therefore, regulation of Nrf2 by nanoparticles is of importance in cancer therapy [246]. However, one of the limitations of current study is lack of significant attention to the properties and type of nanoparticles as well as designing novel nanocarriers and they have only focused on the cargo. Moreover, the specific attention should be directed towards application of nanoenzymes for the suppression of Nrf2 in cancer therapy.
Conclusion
The current challenges for the treatment of cancer are still present and the death of cancer patients results from several factors, mainly metastasis and therapy resistance of tumor cells. Up to 90% of cancer-related deaths are due to the metastasis and invasion of cancer cells. Moreover, cancer metastasis can interfere with the potential of surgical resection in removal of tumor cells. On the other hand, the resistance to therapy has caused problems in the treatment of cancer patients. The mutations, deletions and amplifications occurring in cancer cells can accelerate the progression. The dysregulation of Nrf2 and its amplification have been considered as the main factors in the process of tumorigenesis ad enhancing the progression of cancer. The current paper was allotted to evaluate the potential of Nrf2 in carcinogenesis through regulation of important biological mechanisms in cancer cells. The interaction of Nrf2 with cell death mechanisms is observed in cancer that Nrf2 prevents apoptosis and ferroptosis, while it promotes protective autophagy to ensure tumor progression. Moreover, EMT induction by Nrf2 axis can accelerate the metastasis and invasion of cancer. The overexpression of Nrf2 causes drug resistance and radioresistance that is due to the potential of Nrf2 in increasing survival rate, proliferation and causing cell death resistance in tumor cells. Since radiotherapy causes DNA damage and Nrf2 has association with DNA damage repair, the function of Nrf2 in causing radioresistance is conceivable. Moreover, the suppression of Nrf2 by pharmacological compounds and nanoparticles can disrupt tumor progression. As a result, the function of Nrf2 in cancer is pleiotropic and targeting this pathway can significant suppress the progression of cancer. The benefit of nanoparticles is that they can carry cargo in a targeted way and specifically deliver to the tumor cells for suppression of Nrf2 and impairing cancer progression.
Acknowledgements
The figures of this article was made on Biorender.com.
Disclosure of conflict of interest
None.
References
- 1.Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016;35:75–91. doi: 10.1007/s10555-016-9618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368:eaaw5473. doi: 10.1126/science.aaw5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. 2019;9:217–222. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Q, Kou D, Lou S, Ashrafizadeh M, Aref AR, Canadas I, Tian Y, Niu X, Wang Y, Torabian P, Wang L, Sethi G, Tergaonkar V, Tay F, Yuan Z, Han P. Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy. J Hematol Oncol. 2024;17:16. doi: 10.1186/s13045-024-01535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Liu L, Tian Y, Gu M, Wang Y, Ashrafizadeh M, Reza Aref A, Cañadas I, Klionsky DJ, Goel A, Reiter RJ, Wang Y, Tambuwala M, Zou J. Autophagy-driven regulation of cisplatin response in human cancers: exploring molecular and cell death dynamics. Cancer Lett. 2024;587:216659. doi: 10.1016/j.canlet.2024.216659. [DOI] [PubMed] [Google Scholar]
- 6.Basu AK. DNA damage, mutagenesis and cancer. Int J Mol Sci. 2018;19:970. doi: 10.3390/ijms19040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulis M, Esteller M. DNA methylation and cancer. Adv Genet. 2010;70:27–56. doi: 10.1016/B978-0-12-380866-0.60002-2. [DOI] [PubMed] [Google Scholar]
- 8.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clézardin P, Coleman R, Puppo M, Ottewell P, Bonnelye E, Paycha F, Confavreux CB, Holen I. Bone metastasis: mechanisms, therapies, and biomarkers. Physiol Rev. 2021;101:797–855. doi: 10.1152/physrev.00012.2019. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Chen X, Lin T. Lymphatic metastasis of bladder cancer: molecular mechanisms, diagnosis and targeted therapy. Cancer Lett. 2021;505:13–23. doi: 10.1016/j.canlet.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233–254. doi: 10.3322/caac.21772. [DOI] [PubMed] [Google Scholar]
- 13.Bhinder B, Gilvary C, Madhukar NS, Elemento O. Artificial intelligence in cancer research and precision medicine. Cancer Discov. 2021;11:900–915. doi: 10.1158/2159-8290.CD-21-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa H, Fujita M. Whole genome sequencing analysis for cancer genomics and precision medicine. Cancer Sci. 2018;109:513–522. doi: 10.1111/cas.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Wu H, Chong W, Shang L, Jing C, Li L. Liquid biopsy in gastric cancer: predictive and prognostic biomarkers. Cell Death Dis. 2022;13:903. doi: 10.1038/s41419-022-05350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma S, Zhou M, Xu Y, Gu X, Zou M, Abudushalamu G, Yao Y, Fan X, Wu G. Clinical application and detection techniques of liquid biopsy in gastric cancer. Mol Cancer. 2023;22:7. doi: 10.1186/s12943-023-01715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baranwal J, Barse B, Di Petrillo A, Gatto G, Pilia L, Kumar A. Nanoparticles in cancer diagnosis and treatment. Materials (Basel) 2023;16:5354. doi: 10.3390/ma16155354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng X, Wu Y, Zuo H, Chen W, Wang K. Metal nanoparticles as novel agents for lung cancer diagnosis and therapy. Small. 2023;19:e2206624. doi: 10.1002/smll.202206624. [DOI] [PubMed] [Google Scholar]
- 19.Kunkler IH, Williams LJ, Jack WJL, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in early breast cancer. N Engl J Med. 2023;388:585–594. doi: 10.1056/NEJMoa2207586. [DOI] [PubMed] [Google Scholar]
- 20.Eskander RN, Sill MW, Beffa L, Moore RG, Hope JM, Musa FB, Mannel R, Shahin MS, Cantuaria GH, Girda E, Mathews C, Kavecansky J, Leath CA 3rd, Gien LT, Hinchcliff EM, Lele SB, Landrum LM, Backes F, O’Cearbhaill RE, Al Baghdadi T, Hill EK, Thaker PH, John VS, Welch S, Fader AN, Powell MA, Aghajanian C. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388:2159–2170. doi: 10.1056/NEJMoa2302312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamdy FC, Donovan JL, Lane JA, Metcalfe C, Davis M, Turner EL, Martin RM, Young GJ, Walsh EI, Bryant RJ, Bollina P, Doble A, Doherty A, Gillatt D, Gnanapragasam V, Hughes O, Kockelbergh R, Kynaston H, Paul A, Paez E, Powell P, Rosario DJ, Rowe E, Mason M, Catto JWF, Peters TJ, Oxley J, Williams NJ, Staffurth J, Neal DE ProtecT Study Group. Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2023;388:1547–1558. doi: 10.1056/NEJMoa2214122. [DOI] [PubMed] [Google Scholar]
- 22.Maghnie M, Ranke MB, Geffner ME, Vlachopapadopoulou E, Ibáñez L, Carlsson M, Cutfield W, Rooman R, Gomez R, Wajnrajch MP, Linglart A, Stawerska R, Clayton PE, Darendeliler F, Hokken-Koelega ACS, Horikawa R, Tanaka T, Dörr HG, Albertsson-Wikland K, Polak M, Grimberg A. Safety and efficacy of pediatric growth hormone therapy: results from the full KIGS cohort. J Clin Endocrinol Metab. 2022;107:3287–3301. doi: 10.1210/clinem/dgac517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Lin Z. Non-small cell lung cancer targeted therapy: drugs and mechanisms of drug resistance. Int J Mol Sci. 2022;23:15056. doi: 10.3390/ijms232315056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, Mukherjee A, Paul MK. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22:40. doi: 10.1186/s12943-023-01740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fatemi N, Tierling S, Es HA, Varkiani M, Mojarad EN, Aghdaei HA, Walter J, Totonchi M. DNA methylation biomarkers in colorectal cancer: clinical applications for precision medicine. Int J Cancer. 2022;151:2068–2081. doi: 10.1002/ijc.34186. [DOI] [PubMed] [Google Scholar]
- 26.Aboagye EO, Barwick TD, Haberkorn U. Radiotheranostics in oncology: making precision medicine possible. CA Cancer J Clin. 2023;73:255–274. doi: 10.3322/caac.21768. [DOI] [PubMed] [Google Scholar]
- 27.Rahangdale L, Mungo C, O’Connor S, Chibwesha CJ, Brewer NT. Human papillomavirus vaccination and cervical cancer risk. BMJ. 2022;379:e070115. doi: 10.1136/bmj-2022-070115. [DOI] [PubMed] [Google Scholar]
- 28.Illah O, Olaitan A. Updates on HPV vaccination. Diagnostics (Basel) 2023;13:243. doi: 10.3390/diagnostics13020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devaraja K, Aggarwal S, Singh M. Therapeutic vaccination in head and neck squamous cell carcinoma-a review. Vaccines (Basel) 2023;11:634. doi: 10.3390/vaccines11030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Hussein MT, Dhaliwal S. HPV vaccination for prevention of head and neck cancer among men. Nurse Pract. 2023;48:25–32. doi: 10.1097/01.NPR.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Revach OY, Anderson S, Kessler EA, Wolfe CH, Jenney A, Mills CE, Robitschek EJ, Davis TGR, Kim S, Fu A, Ma X, Gwee J, Tiwari P, Du PP, Sindurakar P, Tian J, Mehta A, Schneider AM, Yizhak K, Sade-Feldman M, LaSalle T, Sharova T, Xie H, Liu S, Michaud WA, Saad-Beretta R, Yates KB, Iracheta-Vellve A, Spetz JKE, Qin X, Sarosiek KA, Zhang G, Kim JW, Su MY, Cicerchia AM, Rasmussen MQ, Klempner SJ, Juric D, Pai SI, Miller DM, Giobbie-Hurder A, Chen JH, Pelka K, Frederick DT, Stinson S, Ivanova E, Aref AR, Paweletz CP, Barbie DA, Sen DR, Fisher DE, Corcoran RB, Hacohen N, Sorger PK, Flaherty KT, Boland GM, Manguso RT, Jenkins RW. Targeting TBK1 to overcome resistance to cancer immunotherapy. Nature. 2023;615:158–167. doi: 10.1038/s41586-023-05704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Tie Y, Alu A, Ma X, Shi H. Targeted therapy for head and neck cancer: signaling pathways and clinical studies. Signal Transduct Target Ther. 2023;8:31. doi: 10.1038/s41392-022-01297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He F, Antonucci L, Karin M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis. 2020;41:405–416. doi: 10.1093/carcin/bgaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taguchi K, Yamamoto M. The KEAP1-NRF2 system in cancer. Front Oncol. 2017;7:85. doi: 10.3389/fonc.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88:101–107. doi: 10.1016/j.freeradbiomed.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 37.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J Biol Chem. 2004;279:31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- 38.Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Liu K, Geng M, Gao P, Wu X, Hai Y, Li Y, Li Y, Luo L, Hayes JD, Wang XJ, Tang X. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013;73:3097–3108. doi: 10.1158/0008-5472.CAN-12-3386. [DOI] [PubMed] [Google Scholar]
- 41.Saha S, Buttari B, Panieri E, Profumo E, Saso L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 2020;25:5474. doi: 10.3390/molecules25225474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Kim YR, Oh JE, Kim MS, Kang MR, Park SW, Han JY, Eom HS, Yoo NJ, Lee SH. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol. 2010;220:446–451. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 46.Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34:21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimta AA, Cenariu D, Irimie A, Magdo L, Nabavi SM, Atanasov AG, Berindan-Neagoe I. The role of Nrf2 activity in cancer development and progression. Cancers (Basel) 2019;11:1755. doi: 10.3390/cancers11111755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Y, Li W, Su ZY, Kong AN. The complexity of the Nrf2 pathway: beyond the antioxidant response. J Nutr Biochem. 2015;26:1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang T, Harder B, Rojo de la Vega M, Wong PK, Chapman E, Zhang DD. p62 links autophagy and Nrf2 signaling. Free Radic Biol Med. 2015;88:199–204. doi: 10.1016/j.freeradbiomed.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain A, Lamark T, Sjøttem E, Larsen KB, Awuh JA, Øvervatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, Zhang DD. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tilija Pun N, Park PH. Role of p62 in the suppression of inflammatory cytokine production by adiponectin in macrophages: involvement of autophagy and p21/Nrf2 axis. Sci Rep. 2017;7:393. doi: 10.1038/s41598-017-00456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, Hoshii T, Hirao A, Takagi K, Mizushima T, Motohashi H, Lee MS, Yoshimori T, Tanaka K, Yamamoto M, Komatsu M. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Moon EJ, Giaccia A. Dual roles of NRF2 in tumor prevention and progression: possible implications in cancer treatment. Free Radic Biol Med. 2015;79:292–299. doi: 10.1016/j.freeradbiomed.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Na HK, Surh YJ. Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic Biol Med. 2014;67:353–365. doi: 10.1016/j.freeradbiomed.2013.10.819. [DOI] [PubMed] [Google Scholar]
- 56.Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, Hayes JD. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med. 2015;88:108–146. doi: 10.1016/j.freeradbiomed.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vomhof-Dekrey EE, Picklo MJ Sr. The Nrf2-antioxidant response element pathway: a target for regulating energy metabolism. J Nutr Biochem. 2012;23:1201–1206. doi: 10.1016/j.jnutbio.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Digaleh H, Kiaei M, Khodagholi F. Nrf2 and Nrf1 signaling and ER stress crosstalk: implication for proteasomal degradation and autophagy. Cell Mol Life Sci. 2013;70:4681–4694. doi: 10.1007/s00018-013-1409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hast BE, Cloer EW, Goldfarb D, Li H, Siesser PF, Yan F, Walter V, Zheng N, Hayes DN, Major MB. Cancer-derived mutations in KEAP1 impair NRF2 degradation but not ubiquitination. Cancer Res. 2014;74:808–817. doi: 10.1158/0008-5472.CAN-13-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi Q, Jin X, Zhang P, Li Q, Lv Z, Ding Y, He H, Wang Y, He Y, Zhao X, Zhao SM, Li Y, Gao K, Wang C. SPOP mutations promote p62/SQSTM1-dependent autophagy and Nrf2 activation in prostate cancer. Cell Death Differ. 2022;29:1228–1239. doi: 10.1038/s41418-021-00913-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kudo Y, Sugimoto M, Arias E, Kasashima H, Cordes T, Linares JF, Duran A, Nakanishi Y, Nakanishi N, L’Hermitte A, Campos A, Senni N, Rooslid T, Roberts LR, Cuervo AM, Metallo CM, Karin M, Diaz-Meco MT, Moscat J. PKCλ/ι loss induces autophagy, oxidative phosphorylation, and NRF2 to promote liver cancer progression. Cancer Cell. 2020;38:247–262. e211. doi: 10.1016/j.ccell.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lignitto L, LeBoeuf SE, Homer H, Jiang S, Askenazi M, Karakousi TR, Pass HI, Bhutkar AJ, Tsirigos A, Ueberheide B, Sayin VI, Papagiannakopoulos T, Pagano M. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of Bach1. Cell. 2019;178:316–329. e318. doi: 10.1016/j.cell.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Q, Zhang ZY, Du H, Li SZ, Tu R, Jia YF, Zheng Z, Song XM, Du RL, Zhang XD. DUB3 deubiquitinates and stabilizes NRF2 in chemotherapy resistance of colorectal cancer. Cell Death Differ. 2019;26:2300–2313. doi: 10.1038/s41418-019-0303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh A, Daemen A, Nickles D, Jeon SM, Foreman O, Sudini K, Gnad F, Lajoie S, Gour N, Mitzner W, Chatterjee S, Choi EJ, Ravishankar B, Rappaport A, Patil N, McCleland M, Johnson L, Acquaah-Mensah G, Gabrielson E, Biswal S, Hatzivassiliou G. NRF2 activation promotes aggressive lung cancer and associates with poor clinical outcomes. Clin Cancer Res. 2021;27:877–888. doi: 10.1158/1078-0432.CCR-20-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiss-Sadan T, Ge M, Hayashi M, Gohar M, Yao CH, de Groot A, Harry S, Carlin A, Fischer H, Shi L, Wei TY, Adelmann CH, Wolf K, Vornbäumen T, Dürr BR, Takahashi M, Richter M, Zhang J, Yang TY, Vijay V, Fisher DE, Hata AN, Haigis MC, Mostoslavsky R, Bardeesy N, Papagiannakopoulos T, Bar-Peled L. NRF2 activation induces NADH-reductive stress, providing a metabolic vulnerability in lung cancer. Cell Metab. 2023;35:487–503. e487. doi: 10.1016/j.cmet.2023.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 68.Feng J, Li J, Wu L, Yu Q, Ji J, Wu J, Dai W, Guo C. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2020;39:126. doi: 10.1186/s13046-020-01629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao L, Kang M, Liu X, Wang Z, Wang Y, Chen H, Liu W, Liu S, Li B, Li C, Chang A, Tang B. UBR7 inhibits HCC tumorigenesis by targeting Keap1/Nrf2/Bach1/HK2 and glycolysis. J Exp Clin Cancer Res. 2022;41:330. doi: 10.1186/s13046-022-02528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu HY, Zhang HS, Liu MY, Li HM, Wang XY, Wang M. GLS1 depletion inhibited colorectal cancer proliferation and migration via redox/Nrf2/autophagy-dependent pathway. Arch Biochem Biophys. 2021;708:108964. doi: 10.1016/j.abb.2021.108964. [DOI] [PubMed] [Google Scholar]
- 71.Zheng XJ, Chen WL, Yi J, Li W, Liu JY, Fu WQ, Ren LW, Li S, Ge BB, Yang YH, Zhang YZ, Yang H, Du GH, Wang Y, Wang JH. Apolipoprotein C1 promotes glioblastoma tumorigenesis by reducing KEAP1/NRF2 and CBS-regulated ferroptosis. Acta Pharmacol Sin. 2022;43:2977–2992. doi: 10.1038/s41401-022-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanghvi VR, Leibold J, Mina M, Mohan P, Berishaj M, Li Z, Miele MM, Lailler N, Zhao C, de Stanchina E, Viale A, Akkari L, Lowe SW, Ciriello G, Hendrickson RC, Wendel HG. The oncogenic action of NRF2 depends on de-glycation by fructosamine-3-kinase. Cell. 2019;178:807–819. e821. doi: 10.1016/j.cell.2019.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang HS, Zhang ZG, Du GY, Sun HL, Liu HY, Zhou Z, Gou XM, Wu XH, Yu XY, Huang YH. Nrf2 promotes breast cancer cell migration via up-regulation of G6PD/HIF-1α/Notch1 axis. J Cell Mol Med. 2019;23:3451–3463. doi: 10.1111/jcmm.14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meng C, Zhan J, Chen D, Shao G, Zhang H, Gu W, Luo J. The deubiquitinase USP11 regulates cell proliferation and ferroptotic cell death via stabilization of NRF2 USP11 deubiquitinates and stabilizes NRF2. Oncogene. 2021;40:1706–1720. doi: 10.1038/s41388-021-01660-5. [DOI] [PubMed] [Google Scholar]
- 75.Tan CT, Chang HC, Zhou Q, Yu C, Fu NY, Sabapathy K, Yu VC. MOAP-1-mediated dissociation of p62/SQSTM1 bodies releases Keap1 and suppresses Nrf2 signaling. EMBO Rep. 2021;22:e50854. doi: 10.15252/embr.202050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li W, Huang G, Wei J, Cao H, Jiang G. ALKBH5 inhibits thyroid cancer progression by promoting ferroptosis through TIAM1-Nrf2/HO-1 axis. Mol Cell Biochem. 2023;478:729–741. doi: 10.1007/s11010-022-04541-x. [DOI] [PubMed] [Google Scholar]
- 77.Sun Q, Ye Z, Qin Y, Fan G, Ji S, Zhuo Q, Xu W, Liu W, Hu Q, Liu M, Zhang Z, Xu X, Yu X. Oncogenic function of TRIM2 in pancreatic cancer by activating ROS-related NRF2/ITGB7/FAK axis. Oncogene. 2020;39:6572–6588. doi: 10.1038/s41388-020-01452-3. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Lu Q, Cai J, Wang Y, Lai X, Qiu Y, Huang Y, Ke Q, Zhang Y, Guan Y, Wu H, Wang Y, Liu X, Shi Y, Zhang K, Wang M, Peng Xiang A. Nestin regulates cellular redox homeostasis in lung cancer through the Keap1-Nrf2 feedback loop. Nat Commun. 2019;10:5043. doi: 10.1038/s41467-019-12925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang X, Lee YH, Jang JH, Kim SJ, Kim SH, Kim DH, Na HK, Kim KO, Baek JH, Surh YJ. ARD1 stabilizes NRF2 through direct interaction and promotes colon cancer progression. Life Sci. 2023;313:121217. doi: 10.1016/j.lfs.2022.121217. [DOI] [PubMed] [Google Scholar]
- 80.Wang K, Wang G, Li G, Zhang W, Wang Y, Lin X, Han C, Chen H, Shi L, Reheman A, Li J, Li Z, Yang X. m6A writer WTAP targets NRF2 to accelerate bladder cancer malignancy via m6A-dependent ferroptosis regulation. Apoptosis. 2023;28:627–638. doi: 10.1007/s10495-023-01817-5. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y, Liu JB, Liu J, Liu M, Liu HL, Zhang J. UCHL3 promotes cervical cancer development and metastasis by stabilizing NRF2 via deubiquitination. Biochem Biophys Res Commun. 2023;641:132–138. doi: 10.1016/j.bbrc.2022.11.065. [DOI] [PubMed] [Google Scholar]
- 82.Chen T, Jinlin D, Wang F, Yuan Z, Xue J, Lu T, Huang W, Liu Y, Zhang Y. GSTM3 deficiency impedes DNA mismatch repair to promote gastric tumorigenesis via CAND1/NRF2-KEAP1 signaling. Cancer Lett. 2022;538:215692. doi: 10.1016/j.canlet.2022.215692. [DOI] [PubMed] [Google Scholar]
- 83.Lee YJ, Kim WI, Bae JH, Cho MK, Lee SH, Nam HS, Choi IH, Cho SW. Overexpression of Nrf2 promotes colon cancer progression via ERK and AKT signaling pathways. Ann Surg Treat Res. 2020;98:159–167. doi: 10.4174/astr.2020.98.4.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mancini MCS, Morelli AP, Severino MB, Pavan ICB, Zambalde ÉP, Góis MM, Silva LGSD, Quintero-Ruiz N, Romeiro CF, Dos Santos DFG, Bezerra RMN, Simabuco FM. Knockout of NRF2 triggers prostate cancer cells death through ROS modulation and sensitizes to cisplatin. J Cell Biochem. 2022;123:2079–2092. doi: 10.1002/jcb.30333. [DOI] [PubMed] [Google Scholar]
- 85.Vartanian S, Lee J, Klijn C, Gnad F, Bagniewska M, Schaefer G, Zhang D, Tan J, Watson SA, Liu L, Chen H, Liang Y, Watanabe C, Cuellar T, Kan D, Hartmaier RJ, Lau T, Costa MR, Martin SE, Merchant M, Haley B, Stokoe D. ERBB3 and IGF1R signaling are required for Nrf2-dependent growth in KEAP1-mutant lung cancer. Cancer Res. 2019;79:4828–4839. doi: 10.1158/0008-5472.CAN-18-2086. [DOI] [PubMed] [Google Scholar]
- 86.Wang X, Zhou T, Yang X, Cao X, Jin G, Zhang P, Guo J, Rong K, Li B, Hu Y, Liu K, Ma P, Qin A, Zhao J. DDRGK1 enhances osteosarcoma chemoresistance via inhibiting KEAP1-mediated NRF2 ubiquitination. Adv Sci (Weinh) 2023;10:e2204438. doi: 10.1002/advs.202204438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dai YQ, Bai Y, Gu J, Fan BY. Stanniocalcin1 knockdown induces ferroptosis and suppresses glycolysis in prostate cancer via the Nrf2 pathway. Neoplasma. 2022;69:1396–1405. doi: 10.4149/neo_2022_220626N665. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, Xin Z, Dong B, Xue W. Combination of the NRF2 inhibitor and autophagy inhibitor significantly inhibited tumorigenicity of castration-resistant prostate cancer. Comput Math Methods Med. 2022;2022:4182401. doi: 10.1155/2022/4182401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lv Y, Lv X, Zhang J, Cao G, Xu C, Zhang B, Lin W. BRD4 targets the KEAP1-Nrf2-G6PD axis and suppresses redox metabolism in small cell lung cancer. Antioxidants (Basel) 2022;11:661. doi: 10.3390/antiox11040661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoshikawa M, Tsuchihashi K, Ishimoto T, Yae T, Motohara T, Sugihara E, Onishi N, Masuko T, Yoshizawa K, Kawashiri S, Mukai M, Asoda S, Kawana H, Nakagawa T, Saya H, Nagano O. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 2013;73:1855–1866. doi: 10.1158/0008-5472.CAN-12-3609-T. [DOI] [PubMed] [Google Scholar]
- 95.Liu DS, Duong CP, Haupt S, Montgomery KG, House CM, Azar WJ, Pearson HB, Fisher OM, Read M, Guerra GR, Haupt Y, Cullinane C, Wiman KG, Abrahmsen L, Phillips WA, Clemons NJ. Inhibiting the system xC-/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat Commun. 2017;8:14844. doi: 10.1038/ncomms14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL, McCormick F, McManus MT. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, Viswanathan SR, Chattopadhyay S, Tamayo P, Yang WS, Rees MG, Chen S, Boskovic ZV, Javaid S, Huang C, Wu X, Tseng YY, Roider EM, Gao D, Cleary JM, Wolpin BM, Mesirov JP, Haber DA, Engelman JA, Boehm JS, Kotz JD, Hon CS, Chen Y, Hahn WC, Levesque MP, Doench JG, Berens ME, Shamji AF, Clemons PA, Stockwell BR, Schreiber SL. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shin D, Kim EH, Lee J, Roh JL. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med. 2018;129:454–462. doi: 10.1016/j.freeradbiomed.2018.10.426. [DOI] [PubMed] [Google Scholar]
- 99.Yang J, Mo J, Dai J, Ye C, Cen W, Zheng X, Jiang L, Ye L. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis. 2021;12:1079. doi: 10.1038/s41419-021-04367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fan Z, Wirth AK, Chen D, Wruck CJ, Rauh M, Buchfelder M, Savaskan N. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6:e371. doi: 10.1038/oncsis.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang C, Zhang Y, Lin S, Liu Y, Li W. Suppressing the KIF20A/NUAK1/Nrf2/GPX4 signaling pathway induces ferroptosis and enhances the sensitivity of colorectal cancer to oxaliplatin. Aging (Albany NY) 2021;13:13515–13534. doi: 10.18632/aging.202774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang H, Huang Q, Xia J, Cheng S, Pei D, Zhang X, Shu X. The E3 ligase MIB1 promotes proteasomal degradation of NRF2 and sensitizes lung cancer cells to ferroptosis. Mol Cancer Res. 2022;20:253–264. doi: 10.1158/1541-7786.MCR-21-0342. [DOI] [PubMed] [Google Scholar]
- 104.Takahashi N, Cho P, Selfors LM, Kuiken HJ, Kaul R, Fujiwara T, Harris IS, Zhang T, Gygi SP, Brugge JS. 3D culture models with CRISPR screens reveal hyperactive NRF2 as a prerequisite for spheroid formation via regulation of proliferation and ferroptosis. Mol Cell. 2020;80:828–844. e826. doi: 10.1016/j.molcel.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai S, Ding Z, Liu X, Zeng J. Trabectedin induces ferroptosis via regulation of HIF-1α/IRP1/TFR1 and Keap1/Nrf2/GPX4 axis in non-small cell lung cancer cells. Chem Biol Interact. 2023;369:110262. doi: 10.1016/j.cbi.2022.110262. [DOI] [PubMed] [Google Scholar]
- 106.Lou JS, Zhao LP, Huang ZH, Chen XY, Xu JT, Tai WC, Tsim KWK, Chen YT, Xie T. Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. Phytomedicine. 2021;80:153370. doi: 10.1016/j.phymed.2020.153370. [DOI] [PubMed] [Google Scholar]
- 107.Liu Y, Huang P, Li Z, Xu C, Wang H, Jia B, Gong A, Xu M. Vitamin C sensitizes pancreatic cancer cells to erastin-induced ferroptosis by activating the AMPK/Nrf2/HMOX1 pathway. Oxid Med Cell Longev. 2022;2022:5361241. doi: 10.1155/2022/5361241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang K, Chen Q, Liu Y, Wang L, Lu W. Combination of metformin and sorafenib induces ferroptosis of hepatocellular carcinoma through p62-Keap1-Nrf2 pathway. J Cancer. 2022;13:3234–3243. doi: 10.7150/jca.76618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guan D, Zhou W, Wei H, Wang T, Zheng K, Yang C, Feng R, Xu R, Fu Y, Li C, Li Y, Li C. Ferritinophagy-mediated ferroptosis and activation of Keap1/Nrf2/HO-1 pathway were conducive to EMT inhibition of gastric cancer cells in action of 2,2’-Di-pyridineketone hydrazone dithiocarbamate butyric acid ester. Oxid Med Cell Longev. 2022;2022:3920664. doi: 10.1155/2022/3920664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hsu WL, Wang CM, Yao CL, Chen SC, Nien CY, Sun YH, Tseng TY, Luo YH. Blockage of Nrf2 and autophagy by L-selenocystine induces selective death in Nrf2-addicted colorectal cancer cells through p62-Keap-1-Nrf2 axis. Cell Death Dis. 2022;13:1060. doi: 10.1038/s41419-022-05512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tao W, Wang N, Ruan J, Cheng X, Fan L, Zhang P, Lu C, Hu Y, Che C, Sun D, Duan J, Zhao M. Enhanced ROS-boosted phototherapy against pancreatic cancer via Nrf2-mediated stress-defense pathway suppression and ferroptosis induction. ACS Appl Mater Interfaces. 2022;14:6404–6416. doi: 10.1021/acsami.1c22861. [DOI] [PubMed] [Google Scholar]
- 112.Wu X, Liu C, Li Z, Gai C, Ding D, Chen W, Hao F, Li W. Regulation of GSK3β/Nrf2 signaling pathway modulated erastin-induced ferroptosis in breast cancer. Mol Cell Biochem. 2020;473:217–228. doi: 10.1007/s11010-020-03821-8. [DOI] [PubMed] [Google Scholar]
- 113.Gai C, Liu C, Wu X, Yu M, Zheng J, Zhang W, Lv S, Li W. MT1DP loaded by folate-modified liposomes sensitizes erastin-induced ferroptosis via regulating miR-365a-3p/NRF2 axis in non-small cell lung cancer cells. Cell Death Dis. 2020;11:751. doi: 10.1038/s41419-020-02939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang S, Yi X, Wu Z, Guo S, Dai W, Wang H, Shi Q, Zeng K, Guo W, Li C. CAMKK2 defines ferroptosis sensitivity of melanoma cells by regulating AMPK-NRF2 pathway. J Invest Dermatol. 2022;142:189–200. e188. doi: 10.1016/j.jid.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 115.Lu Z, Yang H, Shao Y, Sun W, Jiang Y, Li J. IGF2BP3-NRF2 axis regulates ferroptosis in hepatocellular carcinoma. Biochem Biophys Res Commun. 2022;627:103–110. doi: 10.1016/j.bbrc.2022.08.040. [DOI] [PubMed] [Google Scholar]
- 116.Ren X, Li Y, Zhou Y, Hu W, Yang C, Jing Q, Zhou C, Wang X, Hu J, Wang L, Yang J, Wang H, Xu H, Li H, Tong X, Wang Y, Du J. Overcoming the compensatory elevation of NRF2 renders hepatocellular carcinoma cells more vulnerable to disulfiram/copper-induced ferroptosis. Redox Biol. 2021;46:102122. doi: 10.1016/j.redox.2021.102122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Feng H, Liu Y, Gan Y, Li M, Liu R, Liang Z, Liu L, Li L, Chen H, Li G, Tian Z, Liu X, Ma S. AdipoR1 regulates ionizing radiation-induced ferroptosis in HCC cells through Nrf2/xCT pathway. Oxid Med Cell Longev. 2022;2022:8091464. doi: 10.1155/2022/8091464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xia L, Gong M, Zou Y, Wang Z, Wu B, Zhang S, Li L, Jin K, Sun C. Apatinib induces ferroptosis of glioma cells through modulation of the VEGFR2/Nrf2 pathway. Oxid Med Cell Longev. 2022;2022:9925919. doi: 10.1155/2022/9925919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou Y, Chen Y, Shi Y, Wu L, Tan Y, Li T, Chen Y, Xia J, Hu R. FAM117B promotes gastric cancer growth and drug resistance by targeting the KEAP1/NRF2 signaling pathway. J Clin Invest. 2023;133:e158705. doi: 10.1172/JCI158705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deng C, Xiong L, Chen Y, Wu K, Wu J. Metformin induces ferroptosis through the Nrf2/HO-1 signaling in lung cancer. BMC Pulm Med. 2023;23:360. doi: 10.1186/s12890-023-02655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hsieh CH, Kuan WH, Chang WL, Kuo IY, Liu H, Shieh DB, Liu H, Tan B, Wang YC. Dysregulation of SOX17/NRF2 axis confers chemoradiotherapy resistance and emerges as a novel therapeutic target in esophageal squamous cell carcinoma. J Biomed Sci. 2022;29:90. doi: 10.1186/s12929-022-00873-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gao Z, Jiang J, Hou L, Ji F. Lysionotin induces ferroptosis to suppress development of colorectal cancer via promoting Nrf2 degradation. Oxid Med Cell Longev. 2022;2022:1366957. doi: 10.1155/2022/1366957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu X, Peng X, Cen S, Yang C, Ma Z, Shi X. Wogonin induces ferroptosis in pancreatic cancer cells by inhibiting the Nrf2/GPX4 axis. Front Pharmacol. 2023;14:1129662. doi: 10.3389/fphar.2023.1129662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu JF, Liu Y, Li WT, Li MH, Zhen CH, Sun PW, Chen JX, Wu WH, Zeng W. Ibrutinib facilitates the sensitivity of colorectal cancer cells to ferroptosis through BTK/NRF2 pathway. Cell Death Dis. 2023;14:151. doi: 10.1038/s41419-023-05664-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 125.Zhu X, Chen X, Qiu L, Zhu J, Wang J. Norcantharidin induces ferroptosis via the suppression of NRF2/HO-1 signaling in ovarian cancer cells. Oncol Lett. 2022;24:359. doi: 10.3892/ol.2022.13479. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Jing S, Lu Y, Zhang J, Ren Y, Mo Y, Liu D, Duan L, Yuan Z, Wang C, Wang Q. Levistilide a induces ferroptosis by activating the Nrf2/HO-1 signaling pathway in breast cancer cells. Drug Des Devel Ther. 2022;16:2981–2993. doi: 10.2147/DDDT.S374328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun H, Cai H, Xu C, Zhai H, Lux F, Xie Y, Feng L, Du L, Liu Y, Sun X, Wang Q, Song H, He N, Zhang M, Ji K, Wang J, Gu Y, Leduc G, Doussineau T, Wang Y, Liu Q, Tillement O. AGuIX nanoparticles enhance ionizing radiation-induced ferroptosis on tumor cells by targeting the NRF2-GPX4 signaling pathway. J Nanobiotechnology. 2022;20:449. doi: 10.1186/s12951-022-01654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu Y, Pi D, Zhou S, Yi Z, Dong Y, Wang W, Ye H, Chen Y, Zuo Q, Ouyang M. Ginsenoside Rh3 induces pyroptosis and ferroptosis through the Stat3/p53/NRF2 axis in colorectal cancer cells. Acta Biochim Biophys Sin (Shanghai) 2023;55:587–600. doi: 10.3724/abbs.2023068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang X, Chen J, Tie H, Tian W, Zhao Y, Qin L, Guo S, Li Q, Bao C. Eriodictyol regulated ferroptosis, mitochondrial dysfunction, and cell viability via Nrf2/HO-1/NQO1 signaling pathway in ovarian cancer cells. J Biochem Mol Toxicol. 2023;37:e23368. doi: 10.1002/jbt.23368. [DOI] [PubMed] [Google Scholar]
- 130.Feng S, Li Y, Huang H, Huang H, Duan Y, Yuan Z, Zhu W, Mei Z, Luo L, Yan P. Isoorientin reverses lung cancer drug resistance by promoting ferroptosis via the SIRT6/Nrf2/GPX4 signaling pathway. Eur J Pharmacol. 2023;954:175853. doi: 10.1016/j.ejphar.2023.175853. [DOI] [PubMed] [Google Scholar]
- 131.Gai C, Yu M, Li Z, Wang Y, Ding D, Zheng J, Lv S, Zhang W, Li W. Acetaminophen sensitizing erastin-induced ferroptosis via modulation of Nrf2/heme oxygenase-1 signaling pathway in non-small-cell lung cancer. J Cell Physiol. 2020;235:3329–3339. doi: 10.1002/jcp.29221. [DOI] [PubMed] [Google Scholar]
- 132.Hao Q, Chen J, Lu H, Zhou X. The ARTS of p53-dependent mitochondrial apoptosis. J Mol Cell Biol. 2023;14:mjac074. doi: 10.1093/jmcb/mjac074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 134.Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ichim G, Tait SW. A fate worse than death: apoptosis as an oncogenic process. Nat Rev Cancer. 2016;16:539–548. doi: 10.1038/nrc.2016.58. [DOI] [PubMed] [Google Scholar]
- 136.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li H, Zhao J, Zhong XL, Xu PY, Du LJ, Fang P, Tan LJ, Li MJ, Zhang CF, Cao TS. CPLX2 regulates ferroptosis and apoptosis through NRF2 pathway in human hepatocellular carcinoma cells. Appl Biochem Biotechnol. 2023;195:597–609. doi: 10.1007/s12010-022-04135-9. [DOI] [PubMed] [Google Scholar]
- 138.Ruan Q, Wen C, Jin G, Yuan Z, Yang X, Wen Z, Huang G, Li G, Deng J, Bai Y. Phloretin-induced STAT3 inhibition suppresses pancreatic cancer growth and progression via enhancing Nrf2 activity. Phytomedicine. 2023;118:154990. doi: 10.1016/j.phymed.2023.154990. [DOI] [PubMed] [Google Scholar]
- 139.Pandey A, Trigun SK. Fisetin induces apoptosis in colorectal cancer cells by suppressing autophagy and down-regulating nuclear factor erythroid 2-related factor 2 (Nrf2) J Cell Biochem. 2023;124:1289–1308. doi: 10.1002/jcb.30447. [DOI] [PubMed] [Google Scholar]
- 140.Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19:12. doi: 10.1186/s12943-020-1138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qin Y, Ashrafizadeh M, Mongiardini V, Grimaldi B, Crea F, Rietdorf K, Győrffy B, Klionsky DJ, Ren J, Zhang W, Zhang X. Autophagy and cancer drug resistance in dialogue: pre-clinical and clinical evidence. Cancer Lett. 2023;570:216307. doi: 10.1016/j.canlet.2023.216307. [DOI] [PubMed] [Google Scholar]
- 143.Ashrafizadeh M, Zhang W, Zou R, Sethi G, Klionsky DJ, Zhang X. A bioinformatics analysis, pre-clinical and clinical conception of autophagy in pancreatic cancer: complexity and simplicity in crosstalk. Pharmacol Res. 2023;194:106822. doi: 10.1016/j.phrs.2023.106822. [DOI] [PubMed] [Google Scholar]
- 144.Xie C, Zhou X, Liang C, Li X, Ge M, Chen Y, Yin J, Zhu J, Zhong C. Apatinib triggers autophagic and apoptotic cell death via VEGFR2/STAT3/PD-L1 and ROS/Nrf2/p62 signaling in lung cancer. J Exp Clin Cancer Res. 2021;40:266. doi: 10.1186/s13046-021-02069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yu B, Zhou Y, He J. TRIM13 inhibits cell proliferation and induces autophagy in lung adenocarcinoma by regulating KEAP1/NRF2 pathway. Cell Cycle. 2023;22:1496–1513. doi: 10.1080/15384101.2023.2216504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang J, Liu Z, Hu T, Han L, Yu S, Yao Y, Ruan Z, Tian T, Huang T, Wang M, Jing L, Nan K, Liang X. Nrf2 promotes progression of non-small cell lung cancer through activating autophagy. Cell Cycle. 2017;16:1053–1062. doi: 10.1080/15384101.2017.1312224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang Y, Zhang J, Huang ZH, Huang XH, Zheng WB, Yin XF, Li YL, Li B, He QY. Isodeoxyelephantopin induces protective autophagy in lung cancer cells via Nrf2-p62-keap1 feedback loop. Cell Death Dis. 2017;8:e2876. doi: 10.1038/cddis.2017.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu F, Xie Q, Li YW, Jing QQ, Liu XJ, Xu YC, Wang X, Liu L, Kim G, Choi Y, Guo Y, Zhang E, Jin CY. Suppression of JNK/ERK dependent autophagy enhances Jaspine B derivative-induced gastric cancer cell death via attenuation of p62/Keap1/Nrf2 pathways. Toxicol Appl Pharmacol. 2022;438:115908. doi: 10.1016/j.taap.2022.115908. [DOI] [PubMed] [Google Scholar]
- 149.Li X, Liang M, Jiang J, He R, Wang M, Guo X, Shen M, Qin R. Combined inhibition of autophagy and Nrf2 signaling augments bortezomib-induced apoptosis by increasing ROS production and ER stress in pancreatic cancer cells. Int J Biol Sci. 2018;14:1291–1305. doi: 10.7150/ijbs.26776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tang Y, Chen Q, Chen J, Mo Z, Li H, Peng L, Ke Y, Liang B, Li R, Zhu H. Green tea polyphenols cause apoptosis and autophagy in HPV-16 subgene-immortalized human cervical epithelial cells via the activation of the Nrf2 pathway. Nutr Cancer. 2022;74:3769–3778. doi: 10.1080/01635581.2022.2093922. [DOI] [PubMed] [Google Scholar]
- 151.Paik JY, Lee HG, Piao JY, Kim SJ, Kim DH, Na HK, Surh YJ. Helicobacter pylori infection promotes autophagy through Nrf2-mediated heme oxygenase upregulation in human gastric cancer cells. Biochem Pharmacol. 2019;162:89–97. doi: 10.1016/j.bcp.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 152.Lu Z, Ren Y, Yang L, Jia A, Hu Y, Zhao Y, Zhao W, Yu B, Zhao W, Zhang J, Hou G. Inhibiting autophagy enhances sulforaphane-induced apoptosis via targeting NRF2 in esophageal squamous cell carcinoma. Acta Pharm Sin B. 2021;11:1246–1260. doi: 10.1016/j.apsb.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jiang Z, Gao L, Liu C, Wang J, Han Y, Pan J. Sarmentosin induces autophagy-dependent apoptosis via activation of Nrf2 in hepatocellular carcinoma. J Clin Transl Hepatol. 2023;11:863–876. doi: 10.14218/JCTH.2022.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen N, Wu L, Yuan H, Wang J. ROS/Autophagy/Nrf2 pathway mediated low-dose radiation induced radio-resistance in human lung adenocarcinoma A549 cell. Int J Biol Sci. 2015;11:833–844. doi: 10.7150/ijbs.10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao C, Liu W, Deng H, Li J, Ning P, Wang Z. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12:4310–4329. doi: 10.7150/thno.71086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. The interleukin 1beta-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect Immun. 1997;65:5165–5170. doi: 10.1128/iai.65.12.5165-5170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci U S A. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boise LH, Collins CM. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol. 2001;9:64–67. doi: 10.1016/s0966-842x(00)01937-5. [DOI] [PubMed] [Google Scholar]
- 159.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 160.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 161.Fink SL, Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol. 2007;9:2562–2570. doi: 10.1111/j.1462-5822.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- 162.Huang Y, Yang W, Yang L, Wang T, Li C, Yu J, Zhang P, Yin Y, Li R, Tao K. Nrf2 inhibition increases sensitivity to chemotherapy of colorectal cancer by promoting ferroptosis and pyroptosis. Sci Rep. 2023;13:14359. doi: 10.1038/s41598-023-41490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yan J, Wan P, Choksi S, Liu ZG. Necroptosis and tumor progression. Trends Cancer. 2022;8:21–27. doi: 10.1016/j.trecan.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chiu HW, Hung SW, Chiu CF, Hong JR. A mitochondrion-targeting protein (B2) primes ROS/Nrf2-mediated stress signals, triggering apoptosis and necroptosis in lung cancer. Biomedicines. 2023;11:186. doi: 10.3390/biomedicines11010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Babaei G, Aziz SG, Jaghi NZZ. EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed Pharmacother. 2021;133:110909. doi: 10.1016/j.biopha.2020.110909. [DOI] [PubMed] [Google Scholar]
- 166.Akrida I, Papadaki H. Adipokines and epithelial-mesenchymal transition (EMT) in cancer. Mol Cell Biochem. 2023;478:2419–2433. doi: 10.1007/s11010-023-04670-x. [DOI] [PubMed] [Google Scholar]
- 167.Xue W, Yang L, Chen C, Ashrafizadeh M, Tian Y, Sun R. Wnt/β-catenin-driven EMT regulation in human cancers. Cell Mol Life Sci. 2024;81:79. doi: 10.1007/s00018-023-05099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ashrafizadeh M, Dai J, Torabian P, Nabavi N, Aref AR, Aljabali AAA, Tambuwala M, Zhu M. Circular RNAs in EMT-driven metastasis regulation: modulation of cancer cell plasticity, tumorigenesis and therapy resistance. Cell Mol Life Sci. 2024;81:214. doi: 10.1007/s00018-024-05236-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Feng R, Morine Y, Ikemoto T, Imura S, Iwahashi S, Saito Y, Shimada M. Nrf2 activation drive macrophages polarization and cancer cell epithelial-mesenchymal transition during interaction. Cell Commun Signal. 2018;16:54. doi: 10.1186/s12964-018-0262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jin M, Wang J, Ji X, Cao H, Zhu J, Chen Y, Yang J, Zhao Z, Ren T, Xing J. MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:136. doi: 10.1186/s13046-019-1135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cao X, Chen XM, Xiao WZ, Li B, Zhang B, Wu Q, Xue Q. ROS-mediated hypomethylation of PRDX5 promotes STAT3 binding and activates the Nrf2 signaling pathway in NSCLC. Int J Mol Med. 2021;47:573–582. doi: 10.3892/ijmm.2020.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wu YS, Chung I, Wong WF, Masamune A, Sim MS, Looi CY. Paracrine IL-6 signaling mediates the effects of pancreatic stellate cells on epithelial-mesenchymal transition via Stat3/Nrf2 pathway in pancreatic cancer cells. Biochim Biophys Acta Gen Subj. 2017;1861:296–306. doi: 10.1016/j.bbagen.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 173.Hong X, Ma N, Li D, Zhang M, Dong W, Huang J, Ci X, Zhang S. UBE2E2 enhances Snail-mediated epithelial-mesenchymal transition and Nrf2-mediated antioxidant activity in ovarian cancer. Cell Death Dis. 2023;14:100. doi: 10.1038/s41419-023-05636-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fan Q, Liang X, Xu Z, Li S, Han S, Xiao Y, Xu Q, Yuan R, Yang S, Gao H. Pedunculoside inhibits epithelial-mesenchymal transition and overcomes Gefitinib-resistant non-small cell lung cancer through regulating MAPK and Nrf2 pathways. Phytomedicine. 2023;116:154884. doi: 10.1016/j.phymed.2023.154884. [DOI] [PubMed] [Google Scholar]
- 175.Bocci F, Tripathi SC, Vilchez Mercedes SA, George JT, Casabar JP, Wong PK, Hanash SM, Levine H, Onuchic JN, Jolly MK. NRF2 activates a partial epithelial-mesenchymal transition and is maximally present in a hybrid epithelial/mesenchymal phenotype. Integr Biol (Camb) 2019;11:251–263. doi: 10.1093/intbio/zyz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu C, Deng J, Wang S, Ren L. Hypoxia promotes epithelial-mesenchymal transition in lung cancer cells via regulating the NRF2/miR-27a/BUB1 pathway. Clin Transl Oncol. 2023;25:510–522. doi: 10.1007/s12094-022-02965-x. [DOI] [PubMed] [Google Scholar]
- 177.Zhao Q, Mao A, Guo R, Zhang L, Yan J, Sun C, Tang J, Ye Y, Zhang Y, Zhang H. Suppression of radiation-induced migration of non-small cell lung cancer through inhibition of Nrf2-Notch Axis. Oncotarget. 2017;8:36603–36613. doi: 10.18632/oncotarget.16622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Choi BH, Ryu DY, Ryoo IG, Kwak MK. NFE2L2/NRF2 silencing-inducible miR-206 targets c-MET/EGFR and suppresses BCRP/ABCG2 in cancer cells. Oncotarget. 2017;8:107188–107205. doi: 10.18632/oncotarget.22513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhao J, Liu L, Zhao W, Lv C, Zhang N, Jia X, Zhang Z. miR-141-3p accelerates ovarian cancer progression and promotes M2-like macrophage polarization by targeting the Keap1-Nrf2 pathway. Open Med (Wars) 2023;18:20230729. doi: 10.1515/med-2023-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mahajan M, Sitasawad S. miR-140-5p attenuates hypoxia-induced breast cancer progression by targeting Nrf2/HO-1 axis in a Keap1-independent mechanism. Cells. 2021;11:12. doi: 10.3390/cells11010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yin QH, Zhou Y, Li ZHY. miR-373 suppresses cell proliferation and apoptosis via regulation of SIRT1/PGC-1α/NRF2 axis in pancreatic cancer. Cell J. 2021;23:199–210. doi: 10.22074/cellj.2021.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shah NM, Zaitseva L, Bowles KM, MacEwan DJ, Rushworth SA. NRF2-driven miR-125B1 and miR-29B1 transcriptional regulation controls a novel anti-apoptotic miRNA regulatory network for AML survival. Cell Death Differ. 2015;22:654–664. doi: 10.1038/cdd.2014.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gu S, Lai Y, Chen H, Liu Y, Zhang Z. miR-155 mediates arsenic trioxide resistance by activating Nrf2 and suppressing apoptosis in lung cancer cells. Sci Rep. 2017;7:12155. doi: 10.1038/s41598-017-06061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang ZH, Wang Y, Zhang Y, Zheng SF, Feng T, Tian X, Abudurexiti M, Wang ZD, Zhu WK, Su JQ, Zhang HL, Shi GH, Wang ZL, Cao DL, Ye DW. The function and mechanisms of action of circular RNAs in urologic cancer. Mol Cancer. 2023;22:61. doi: 10.1186/s12943-023-01766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liao W, Feng Q, Liu H, Du J, Chen X, Zeng Y. Circular RNAs in cholangiocarcinoma. Cancer Lett. 2023;553:215980. doi: 10.1016/j.canlet.2022.215980. [DOI] [PubMed] [Google Scholar]
- 186.Cai ZR, Hu Y, Liao K, Li H, Chen DL, Ju HQ. Circular RNAs: emerging regulators of glucose metabolism in cancer. Cancer Lett. 2023;552:215978. doi: 10.1016/j.canlet.2022.215978. [DOI] [PubMed] [Google Scholar]
- 187.Wang Y, Ren F, Sun D, Liu J, Liu B, He Y, Pang S, Shi B, Zhou F, Yao L, Lang Y, Xu S, Wang J. CircKEAP1 suppresses the progression of lung adenocarcinoma via the miR-141-3p/KEAP1/NRF2 axis. Front Oncol. 2021;11:672586. doi: 10.3389/fonc.2021.672586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li M, Zhao Y, Li H, Deng X, Sheng M. Application value of circulating LncRNA in diagnosis, treatment, and prognosis of breast cancer. Funct Integr Genomics. 2023;23:61. doi: 10.1007/s10142-023-00983-8. [DOI] [PubMed] [Google Scholar]
- 189.Zhong C, Xie Z, Duan S. H1Innovative approaches to combat anti-cancer drug resistance: targeting lncRNA and autophagy. Clin Transl Med. 2023;13:e1445. doi: 10.1002/ctm2.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hou J, Zhang G, Wang X, Wang Y, Wang K. Functions and mechanisms of lncRNA MALAT1 in cancer chemotherapy resistance. Biomark Res. 2023;11:23. doi: 10.1186/s40364-023-00467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang T, Liang S, Li Y, Wang X, Wang H, Guo J, Li M. Downregulation of lncRNA SLC7A11-AS1 decreased the NRF2/SLC7A11 expression and inhibited the progression of colorectal cancer cells. PeerJ. 2023;11:e15216. doi: 10.7717/peerj.15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Luo P, Wu S, Ji K, Yuan X, Li H, Chen J, Tian Y, Qiu Y, Zhong X. LncRNA MIR4435-2HG mediates cisplatin resistance in HCT116 cells by regulating Nrf2 and HO-1. PLoS One. 2020;15:e0223035. doi: 10.1371/journal.pone.0223035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Eades G, Yang M, Yao Y, Zhang Y, Zhou Q. miR-200a regulates Nrf2 activation by targeting Keap1 mRNA in breast cancer cells. J Biol Chem. 2011;286:40725–40733. doi: 10.1074/jbc.M111.275495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yi J, Huang WZ, Wen YQ, Yi YC. Effect of miR-101 on proliferation and oxidative stress-induced apoptosis of breast cancer cells via Nrf2 signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:8931–8939. doi: 10.26355/eurrev_201910_19291. [DOI] [PubMed] [Google Scholar]
- 195.Yin Y, Liu H, Xu J, Shi D, Zhai L, Liu B, Wang L, Liu G, Qin J. miR-144-3p regulates the resistance of lung cancer to cisplatin by targeting Nrf2. Oncol Rep. 2018;40:3479–3488. doi: 10.3892/or.2018.6772. [DOI] [PubMed] [Google Scholar]
- 196.Xiao S, Liu N, Yang X, Ji G, Li M. Polygalacin D suppresses esophageal squamous cell carcinoma growth and metastasis through regulating miR-142-5p/Nrf2 axis. Free Radic Biol Med. 2021;164:58–75. doi: 10.1016/j.freeradbiomed.2020.11.029. [DOI] [PubMed] [Google Scholar]
- 197.Bai X, Shao J, Duan T, Liu X, Wang M, Li X, You Q, Zhang Z, Pan J. Exo-miR-1290-induced by COX-2 overexpression promotes cancer-associated fibroblasts activation and tumor progression by CUL3-Nrf2 pathway in lung adenocarcinoma. Cell Commun Signal. 2023;21:242. doi: 10.1186/s12964-023-01268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Han M, Li N, Li F, Wang H, Ma L. MiR-27b-3p exerts tumor suppressor effects in esophageal squamous cell carcinoma by targeting Nrf2. Hum Cell. 2020;33:641–651. doi: 10.1007/s13577-020-00329-7. [DOI] [PubMed] [Google Scholar]
- 199.Singh B, Ronghe AM, Chatterjee A, Bhat NK, Bhat HK. MicroRNA-93 regulates NRF2 expression and is associated with breast carcinogenesis. Carcinogenesis. 2013;34:1165–1172. doi: 10.1093/carcin/bgt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu LL, Cai WP, Lei X, Shi KQ, Lin XY, Shi L. NRAL mediates cisplatin resistance in hepatocellular carcinoma via miR-340-5p/Nrf2 axis. J Cell Commun Signal. 2019;13:99–112. doi: 10.1007/s12079-018-0479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gao AM, Zhang XY, Ke ZP. Apigenin sensitizes BEL-7402/ADM cells to doxorubicin through inhibiting miR-101/Nrf2 pathway. Oncotarget. 2017;8:82085–82091. doi: 10.18632/oncotarget.18294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shi L, Chen ZG, Wu LL, Zheng JJ, Yang JR, Chen XF, Chen ZQ, Liu CL, Chi SY, Zheng JY, Huang HX, Lin XY, Zheng F. miR-340 reverses cisplatin resistance of hepatocellular carcinoma cell lines by targeting Nrf2-dependent antioxidant pathway. Asian Pac J Cancer Prev. 2014;15:10439–10444. doi: 10.7314/apjcp.2014.15.23.10439. [DOI] [PubMed] [Google Scholar]
- 203.Shi L, Wu L, Chen Z, Yang J, Chen X, Yu F, Zheng F, Lin X. MiR-141 activates Nrf2-dependent antioxidant pathway via down-regulating the expression of Keap1 conferring the resistance of hepatocellular carcinoma cells to 5-fluorouracil. Cell Physiol Biochem. 2015;35:2333–2348. doi: 10.1159/000374036. [DOI] [PubMed] [Google Scholar]
- 204.Sun X, Liu D, Xue Y, Hu X. Enforced miR-144-3p expression as a non-invasive biomarker for the acute myeloid leukemia patients mainly by targeting NRF2. Clin Lab. 2017;63:679–687. doi: 10.7754/Clin.Lab.2016.161116. [DOI] [PubMed] [Google Scholar]
- 205.Wu H, Liu N, He A, Li H, Liu H, Qian J, Mao W, Fu G. LMNTD2-AS1 regulates immune cell infiltration and promotes prostate cancer progression by targeting FUS to regulate NRF2 signal pathway. Am J Cancer Res. 2023;13:3384–3400. [PMC free article] [PubMed] [Google Scholar]
- 206.Yang G, Yin H, Lin F, Gao S, Zhan K, Tong H, Tang X, Pan Q, Gou X. Long noncoding RNA TUG1 regulates prostate cancer cell proliferation, invasion and migration via the Nrf2 signaling axis. Pathol Res Pract. 2020;216:152851. doi: 10.1016/j.prp.2020.152851. [DOI] [PubMed] [Google Scholar]
- 207.Zhang Z, Xiong R, Li C, Xu M, Guo M. LncRNA TUG1 promotes cisplatin resistance in esophageal squamous cell carcinoma cells by regulating Nrf2. Acta Biochim Biophys Sin (Shanghai) 2019;51:826–833. doi: 10.1093/abbs/gmz069. [DOI] [PubMed] [Google Scholar]
- 208.Han Y, Gao X, Wu N, Jin Y, Zhou H, Wang W, Liu H, Chu Y, Cao J, Jiang M, Yang S, Shi Y, Xie X, Chen F, Han Y, Qin W, Xu B, Liang J. Long noncoding RNA LINC00239 inhibits ferroptosis in colorectal cancer by binding to Keap1 to stabilize Nrf2. Cell Death Dis. 2022;13:742. doi: 10.1038/s41419-022-05192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhou X, An B, Lin Y, Ni Y, Zhao X, Liang X. Molecular mechanisms of ROS-modulated cancer chemoresistance and therapeutic strategies. Biomed Pharmacother. 2023;165:115036. doi: 10.1016/j.biopha.2023.115036. [DOI] [PubMed] [Google Scholar]
- 210.Eptaminitaki GC, Stellas D, Bonavida B, Baritaki S. Long non-coding RNAs (lncRNAs) signaling in cancer chemoresistance: from prediction to druggability. Drug Resist Updat. 2022;65:100866. doi: 10.1016/j.drup.2022.100866. [DOI] [PubMed] [Google Scholar]
- 211.Assaraf YG, Brozovic A, Gonçalves AC, Jurkovicova D, Linē A, Machuqueiro M, Saponara S, Sarmento-Ribeiro AB, Xavier CPR, Vasconcelos MH. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist Updat. 2019;46:100645. doi: 10.1016/j.drup.2019.100645. [DOI] [PubMed] [Google Scholar]
- 212.Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020;21:3233. doi: 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Taylor S, Spugnini EP, Assaraf YG, Azzarito T, Rauch C, Fais S. Microenvironment acidity as a major determinant of tumor chemoresistance: proton pump inhibitors (PPIs) as a novel therapeutic approach. Drug Resist Updat. 2015;23:69–78. doi: 10.1016/j.drup.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 214.Andrei L, Kasas S, Ochoa Garrido I, Stanković T, Suárez Korsnes M, Vaclavikova R, Assaraf YG, Pešić M. Advanced technological tools to study multidrug resistance in cancer. Drug Resist Updat. 2020;48:100658. doi: 10.1016/j.drup.2019.100658. [DOI] [PubMed] [Google Scholar]
- 215.Haider T, Pandey V, Banjare N, Gupta PN, Soni V. Drug resistance in cancer: mechanisms and tackling strategies. Pharmacol Rep. 2020;72:1125–1151. doi: 10.1007/s43440-020-00138-7. [DOI] [PubMed] [Google Scholar]
- 216.Hussain S, Singh A, Nazir SU, Tulsyan S, Khan A, Kumar R, Bashir N, Tanwar P, Mehrotra R. Cancer drug resistance: a fleet to conquer. J Cell Biochem. 2019;120:14213–14225. doi: 10.1002/jcb.28782. [DOI] [PubMed] [Google Scholar]
- 217.Xie Y, Feng SL, He F, Yan PY, Yao XJ, Fan XX, Leung EL, Zhou H. Down-regulating Nrf2 by tangeretin reverses multiple drug resistance to both chemotherapy and EGFR tyrosine kinase inhibitors in lung cancer. Pharmacol Res. 2022;186:106514. doi: 10.1016/j.phrs.2022.106514. [DOI] [PubMed] [Google Scholar]
- 218.Osman AA, Arslan E, Bartels M, Michikawa C, Lindemann A, Tomczak K, Yu W, Sandulache V, Ma W, Shen L, Wang J, Singh AK, Frederick MJ, Spencer ND, Kovacs J, Heffernan T, Symmans WF, Rai K, Myers JN. Dysregulation and epigenetic reprogramming of NRF2 signaling axis promote acquisition of cisplatin resistance and metastasis in head and neck squamous cell carcinoma. Clin Cancer Res. 2023;29:1344–1359. doi: 10.1158/1078-0432.CCR-22-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cui J, Guo Y, Yin T, Gou S, Xiong J, Liang X, Lu C, Peng T. USP8 promotes gemcitabine resistance of pancreatic cancer via deubiquitinating and stabilizing Nrf2. Biomed Pharmacother. 2023;166:115359. doi: 10.1016/j.biopha.2023.115359. [DOI] [PubMed] [Google Scholar]
- 220.Tian L, Peng Y, Yang K, Cao J, Du X, Liang Z, Shi J, Zhang J. The ERα-NRF2 signalling axis promotes bicalutamide resistance in prostate cancer. Cell Commun Signal. 2022;20:178. doi: 10.1186/s12964-022-00979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liang C, Zhang HY, Wang YQ, Yang LA, Du YS, Luo Y, Zhang TC, Xu Y. TMED2 induces cisplatin resistance in breast cancer via targeting the KEAP1-Nrf2 pathway. Curr Med Sci. 2023;43:1023–1032. doi: 10.1007/s11596-023-2777-7. [DOI] [PubMed] [Google Scholar]
- 222.Xu T, Yang Y, Chen Z, Wang J, Wang X, Zheng Y, Wang C, Wang Y, Zhu Z, Ding X, Zhou J, Li G, Zhang H, Zhang W, Wu Y, Song X. TNFAIP2 confers cisplatin resistance in head and neck squamous cell carcinoma via KEAP1/NRF2 signaling. J Exp Clin Cancer Res. 2023;42:190. doi: 10.1186/s13046-023-02775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chang K, Chen Y, Zhang X, Zhang W, Xu N, Zeng B, Wang Y, Feng T, Dai B, Xu F, Ye D, Wang C. DPP9 stabilizes NRF2 to suppress ferroptosis and induce sorafenib resistance in clear cell renal cell carcinoma. Cancer Res. 2023;83:3940–3955. doi: 10.1158/0008-5472.CAN-22-4001. [DOI] [PubMed] [Google Scholar]
- 224.Zhao J, Hou M, Ding K, Li S, Li H, Zhang X, Bai Z, Liu W. Jie Geng Tang reverses cisplatin resistance through the Nrf2 pathway in lung cancer. J Pharm Pharmacol. 2023;75:784–805. doi: 10.1093/jpp/rgad018. [DOI] [PubMed] [Google Scholar]
- 225.Kim JW, Kim MJ, Han TH, Lee JY, Kim S, Kim H, Oh KJ, Kim WK, Han BS, Bae KH, Ban HS, Bae SH, Lee SC, Lee H, Lee EW. FSP1 confers ferroptosis resistance in KEAP1 mutant non-small cell lung carcinoma in NRF2-dependent and -independent manner. Cell Death Dis. 2023;14:567. doi: 10.1038/s41419-023-06070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Meng Y, Lin W, Wang N, Wei X, Huang Q, Liao Y. Bazedoxifene-induced ROS promote mitochondrial dysfunction and enhance osimertinib sensitivity by inhibiting the p-STAT3/SOCS3 and KEAP1/NRF2 pathways in non-small cell lung cancer. Free Radic Biol Med. 2023;196:65–80. doi: 10.1016/j.freeradbiomed.2023.01.004. [DOI] [PubMed] [Google Scholar]
- 227.Shao W, Wang X, Liu Z, Song X, Wang F, Liu X, Yu Z. Cyperotundone combined with adriamycin induces apoptosis in MCF-7 and MCF-7/ADR cancer cells by ROS generation and NRF2/ARE signaling pathway. Sci Rep. 2023;13:1384. doi: 10.1038/s41598-022-26767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wu Q, Zhang H, You S, Xu Z, Liu X, Chen X, Zhang W, Ye J, Li P, Zhou X. NEDD4L inhibits migration, invasion, cisplatin resistance and promotes apoptosis of bladder cancer cells by inactivating the p62/Keap1/Nrf2 pathway. Environ Toxicol. 2023;38:1678–1689. doi: 10.1002/tox.23796. [DOI] [PubMed] [Google Scholar]
- 229.Ma B, Zhong Y, Chen R, Zhan X, Huang G, Xiong Y, Tan B. Tripterygium glycosides reverse chemotherapy resistance in ovarian cancer by targeting the NRF2/GPX4 signal axis to induce ferroptosis of drug-resistant human epithelial ovarian cancer cells. Biochem Biophys Res Commun. 2023;665:178–186. doi: 10.1016/j.bbrc.2023.04.111. [DOI] [PubMed] [Google Scholar]
- 230.Wu X, Chen S, Huang K, Lin G. Triptolide promotes ferroptosis by suppressing Nrf2 to overcome leukemia cell resistance to doxorubicin. Mol Med Rep. 2023;27:17. doi: 10.3892/mmr.2022.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wei R, Zhao Y, Wang J, Yang X, Li S, Wang Y, Yang X, Fei J, Hao X, Zhao Y, Gui L, Ding X. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int J Biol Sci. 2021;17:2703–2717. doi: 10.7150/ijbs.59404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yang C, Wang T, Zhao Y, Meng X, Ding W, Wang Q, Liu C, Deng H. Flavonoid 4,4’-dimethoxychalcone induced ferroptosis in cancer cells by synergistically activating Keap1/Nrf2/HMOX1 pathway and inhibiting FECH. Free Radic Biol Med. 2022;188:14–23. doi: 10.1016/j.freeradbiomed.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 233.Li S, Zhang Y, Zhang J, Yu B, Wang W, Jia B, Chang J, Liu J. Neferine exerts ferroptosis-inducing effect and antitumor effect on thyroid cancer through Nrf2/HO-1/NQO1 inhibition. J Oncol. 2022;2022:7933775. doi: 10.1155/2022/7933775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tsai KJ, Tsai HY, Tsai CC, Chen TY, Hsieh TH, Chen CL, Mbuyisa L, Huang YB, Lin MW. Luteolin inhibits breast cancer stemness and enhances chemosensitivity through the Nrf2-mediated pathway. Molecules. 2021;26:6452. doi: 10.3390/molecules26216452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hu M, Sun D, Yu J, Fu Y, Qin Z, Huang B, Zhang Q, Chen X, Wei Y, Zhu H, Wang Y, Feng Y, Zheng W, Liao H, Li J, Wu S, Zhang Z. Brusatol sensitizes endometrial hyperplasia and cancer to progestin by suppressing NRF2-TET1-AKR1C1-mediated progestin metabolism. Lab Invest. 2022;102:1335–1345. doi: 10.1038/s41374-022-00816-5. [DOI] [PubMed] [Google Scholar]
- 236.Zhang B, Hou Q, Zhang X, Ma Y, Yuan J, Li S, Zhao X, Sun L, Wang H, Zheng H. Anesthetic propofol inhibits ferroptosis and aggravates distant cancer metastasis via Nrf2 upregulation. Free Radic Biol Med. 2023;195:298–308. doi: 10.1016/j.freeradbiomed.2022.12.092. [DOI] [PubMed] [Google Scholar]
- 237.Lu Q. Bioresponsive and multifunctional cyclodextrin-based non-viral nanocomplexes in cancer therapy: building foundations for gene and drug delivery, immunotherapy and bioimaging. Environ Res. 2023;234:116507. doi: 10.1016/j.envres.2023.116507. [DOI] [PubMed] [Google Scholar]
- 238.Alqurashi YE, Al-Hetty HRAK, Ramaiah P, Fazaa AH, Jalil AT, Alsaikhan F, Gupta J, Ramírez-Coronel AA, Tayyib NA, Peng H. Harnessing function of EMT in hepatocellular carcinoma: from biological view to nanotechnological standpoint. Environ Res. 2023;227:115683. doi: 10.1016/j.envres.2023.115683. [DOI] [PubMed] [Google Scholar]
- 239.Saleem HM, Ramaiah P, Gupta J, Jalil AT, Kadhim NA, Alsaikhan F, Ramírez-Coronel AA, Tayyib NA, Guo Q. Nanotechnology-empowered lung cancer therapy: from EMT role in cancer metastasis to application of nanoengineered structures for modulating growth and metastasis. Environ Res. 2023;232:115942. doi: 10.1016/j.envres.2023.115942. [DOI] [PubMed] [Google Scholar]
- 240.He P, Dai Q, Wu X. New insight in urological cancer therapy: from epithelial-mesenchymal transition (EMT) to application of nano-biomaterials. Environ Res. 2023;229:115672. doi: 10.1016/j.envres.2023.115672. [DOI] [PubMed] [Google Scholar]
- 241.Argenziano M, Bessone F, Dianzani C, Cucci MA, Grattarola M, Pizzimenti S, Cavalli R. Ultrasound-responsive Nrf2-targeting siRNA-loaded nanobubbles for enhancing the treatment of melanoma. Pharmaceutics. 2022;14:341. doi: 10.3390/pharmaceutics14020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Almajidi YQ, Kadhim MM, Alsaikhan F, Turki Jalil A, Hassan Sayyid N, Alexis Ramírez-Coronel A, Hassan Jawhar Z, Gupta J, Nabavi N, Yu W, Ertas YN. Doxorubicin-loaded micelles in tumor cell-specific chemotherapy. Environ Res. 2023;227:115722. doi: 10.1016/j.envres.2023.115722. [DOI] [PubMed] [Google Scholar]
- 243.Zhao Y, Xiao W, Peng W, Huang Q, Wu K, Evans CE, Liu X, Jin H. Oridonin-loaded nanoparticles inhibit breast cancer progression through regulation of ROS-related Nrf2 signaling pathway. Front Bioeng Biotechnol. 2021;9:600579. doi: 10.3389/fbioe.2021.600579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Raj RK, D E, S R. β-Sitosterol-assisted silver nanoparticles activates Nrf2 and triggers mitochondrial apoptosis via oxidative stress in human hepatocellular cancer cell line. J Biomed Mater Res A. 2020;108:1899–1908. doi: 10.1002/jbm.a.36953. [DOI] [PubMed] [Google Scholar]
- 245.Hsieh CH, Hsieh HC, Shih FS, Wang PW, Yang LX, Shieh DB, Wang YC. An innovative NRF2 nano-modulator induces lung cancer ferroptosis and elicits an immunostimulatory tumor microenvironment. Theranostics. 2021;11:7072–7091. doi: 10.7150/thno.57803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Velavan B, Divya T, Sureshkumar A, Sudhandiran G. Nano-chemotherapeutic efficacy of (-) -epigallocatechin 3-gallate mediating apoptosis in A549 cells: involvement of reactive oxygen species mediated Nrf2/Keap1signaling. Biochem Biophys Res Commun. 2018;503:1723–1731. doi: 10.1016/j.bbrc.2018.07.105. [DOI] [PubMed] [Google Scholar]