Abstract
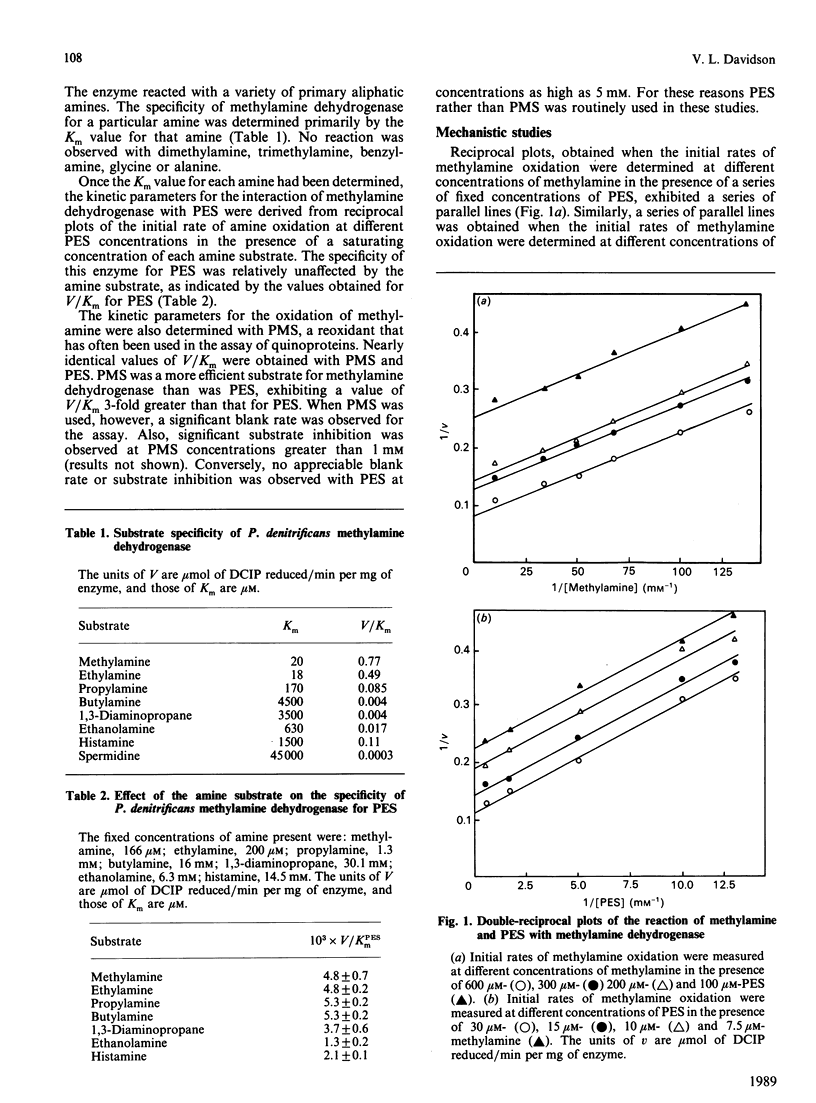
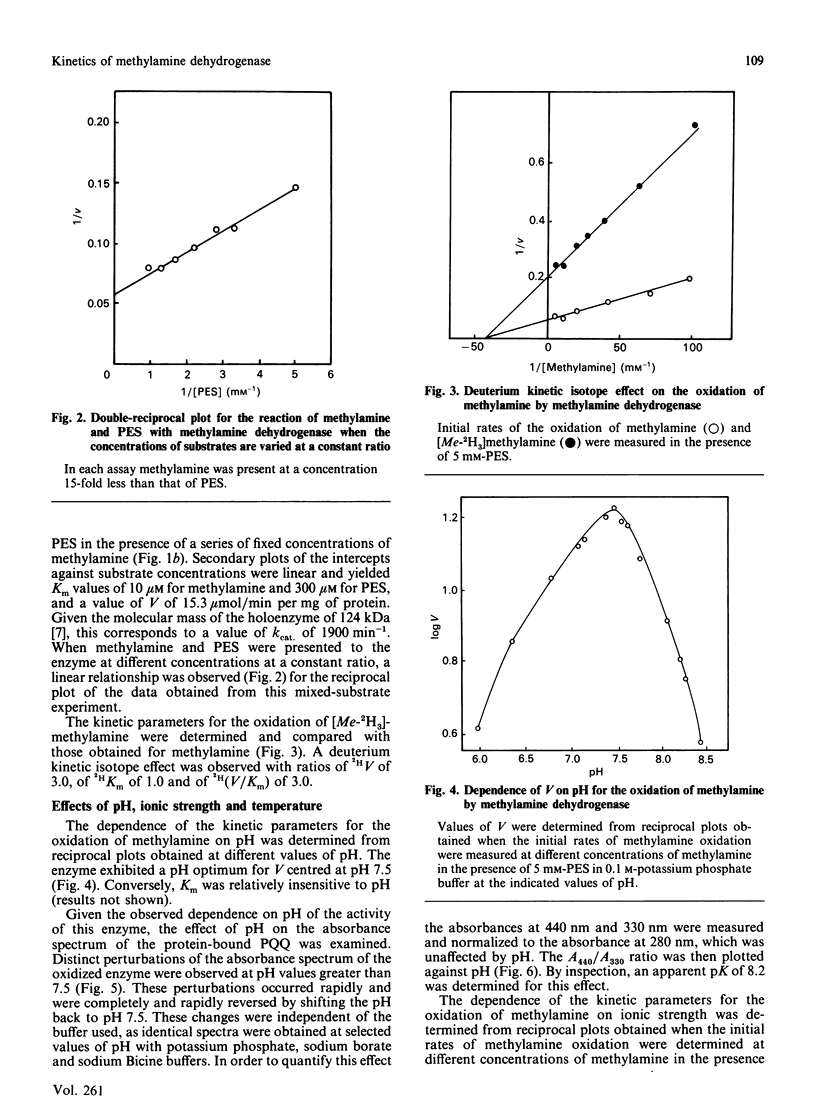
A steady-state kinetic analysis was performed of the reaction of methylamine and phenazine ethosulphate (PES) with the quinoprotein methylamine dehydrogenase from Paracoccus denitrificans. Experiments with methylamine and PES as varied-concentration substrates produced a series of parallel reciprocal plots, and when the concentrations of these substrates were varied in a constant ratio a linear reciprocal plot of initial velocity against PES concentration was obtained. Nearly identical values of V/Km of PES were obtained with four different n-alkylamines. These data suggest that this reaction proceeds by a ping-pong type of mechanism. The enzyme reacted with a variety of n-alkylamines but not with secondary, tertiary or aromatic amines or amino acids. The substrate specificity was dictated primarily by the Km value exhibited by the particular amine. A deuterium kinetic isotope effect was observed with deuterated methylamine as a substrate. The enzyme exhibited a pH optimum for V at pH 7.5. The absorbance spectrum of the pyrroloquinoline quinone prosthetic group of this enzyme was also effected by pH at values greater than 7.5. The enzyme was relatively insensitive to changes in ionic strength, and exhibited a linear Arrhenius plot over a range of temperatures from 10 degrees C to 50 degrees C with an energy of activation 46 kJ/mol (11 kcal/mol).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Bardsley W. G., Crabbe M. J., Shindler J. S. Kinetics of the diamine oxidase reaction. Biochem J. 1973 Mar;131(3):459–469. doi: 10.1042/bj1310459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Chen L., Lim L. W., Mathews F. S., Davidson V. L., Husain M. Preliminary X-ray crystallographic studies of methylamine dehydrogenase and methylamine dehydrogenase--amicyanin complexes from Paracoccus denitrificans. J Mol Biol. 1988 Oct 20;203(4):1137–1138. doi: 10.1016/0022-2836(88)90134-9. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Jr The prosthetic group of methanol dehydrogenase. Purification and some of its properties. Biochem J. 1980 Apr 1;187(1):221–226. doi: 10.1042/bj1870221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duine J. A., Frank J., van Zeeland J. K. Glucose dehydrogenase from Acinetobacter calcoaceticus: a 'quinoprotein'. FEBS Lett. 1979 Dec 15;108(2):443–446. doi: 10.1016/0014-5793(79)80584-0. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Microbial oxidation of amines. Spectral and kinetic properties of the primary amine dehydrogenase of Pseudomonas AM1. Biochem J. 1971 Aug;123(5):757–771. doi: 10.1042/bj1230757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem J. 1968 Jan;106(1):245–255. doi: 10.1042/bj1060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K. A., Davidson V. L., Knaff D. B. Complex formation between methylamine dehydrogenase and amicyanin from Paracoccus denitrificans. J Biol Chem. 1988 Oct 5;263(28):13987–13990. [PubMed] [Google Scholar]
- Hartmann C., Klinman J. P. Pyrroloquinoline quinone: a new redox cofactor in eukaryotic enzymes. Biofactors. 1988 Jan;1(1):41–49. [PubMed] [Google Scholar]
- Husain M., Davidson V. L. An inducible periplasmic blue copper protein from Paracoccus denitrificans. Purification, properties, and physiological role. J Biol Chem. 1985 Nov 25;260(27):14626–14629. [PubMed] [Google Scholar]
- Husain M., Davidson V. L., Gray K. A., Knaff D. B. Redox properties of the quinoprotein methylamine dehydrogenase from paracoccus denitrificans. Biochemistry. 1987 Jun 30;26(13):4139–4143. doi: 10.1021/bi00387a059. [DOI] [PubMed] [Google Scholar]
- Husain M., Davidson V. L. Purification and properties of methylamine dehydrogenase from Paracoccus denitrificans. J Bacteriol. 1987 Apr;169(4):1712–1717. doi: 10.1128/jb.169.4.1712-1717.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney W. C., McIntire W. Characterization of methylamine dehydrogenase from bacterium W3A1. Interaction with reductants and amino-containing compounds. Biochemistry. 1983 Aug 2;22(16):3858–3868. doi: 10.1021/bi00285a022. [DOI] [PubMed] [Google Scholar]
- Lobenstein-Verbeek C. L., Jongejan J. A., Frank J., Duine J. A. Bovine serum amine oxidase: a mammalian enzyme having covalently bound PQQ as prosthetic group. FEBS Lett. 1984 May 21;170(2):305–309. doi: 10.1016/0014-5793(84)81333-2. [DOI] [PubMed] [Google Scholar]
- Moog R. S., McGuirl M. A., Cote C. E., Dooley D. M. Evidence for methoxatin (pyrroloquinolinequinone) as the cofactor in bovine plasma amine oxidase from resonance Raman spectroscopy. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8435–8439. doi: 10.1073/pnas.83.22.8435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palcic M. M., Klinman J. P. Isotopic probes yield microscopic constants: separation of binding energy from catalytic efficiency in the bovine plasma amine oxidase reaction. Biochemistry. 1983 Dec 6;22(25):5957–5966. doi: 10.1021/bi00294a040. [DOI] [PubMed] [Google Scholar]
- Rius F. X., Knowles P. F., Pettersson G. The kinetics of ammonia release during the catalytic cycle of pig plasma amine oxidase. Biochem J. 1984 Jun 15;220(3):767–772. doi: 10.1042/bj2200767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer M. G., Davidson G. P., Goodwin D., Crettenden A. D. Recurrent abdominal pain in childhood. Relationship to psychological adjustment of children and families: a preliminary study. Aust Paediatr J. 1987 Apr;23(2):121–124. doi: 10.1111/j.1440-1754.1987.tb02191.x. [DOI] [PubMed] [Google Scholar]
- Williamson P. R., Kagan H. M. Reaction pathway of bovine aortic lysyl oxidase. J Biol Chem. 1986 Jul 15;261(20):9477–9482. [PubMed] [Google Scholar]
- Williamson P. R., Moog R. S., Dooley D. M., Kagan H. M. Evidence for pyrroloquinolinequinone as the carbonyl cofactor in lysyl oxidase by absorption and resonance Raman spectroscopy. J Biol Chem. 1986 Dec 15;261(35):16302–16305. [PubMed] [Google Scholar]
- de Beer R., Duine J. A., Frank J., Large P. J. The prosthetic group of methylamine dehydrogenase from Pseudomonas AM1: evidence for a quinone structure. Biochim Biophys Acta. 1980 Apr 25;622(2):370–374. doi: 10.1016/0005-2795(80)90050-1. [DOI] [PubMed] [Google Scholar]
- van der Meer R. A., Duine J. A. Covalently bound pyrroloquinoline quinone is the organic prosthetic group in human placental lysyl oxidase. Biochem J. 1986 Nov 1;239(3):789–791. doi: 10.1042/bj2390789. [DOI] [PMC free article] [PubMed] [Google Scholar]


