Abstract
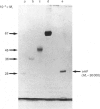
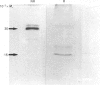
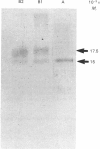
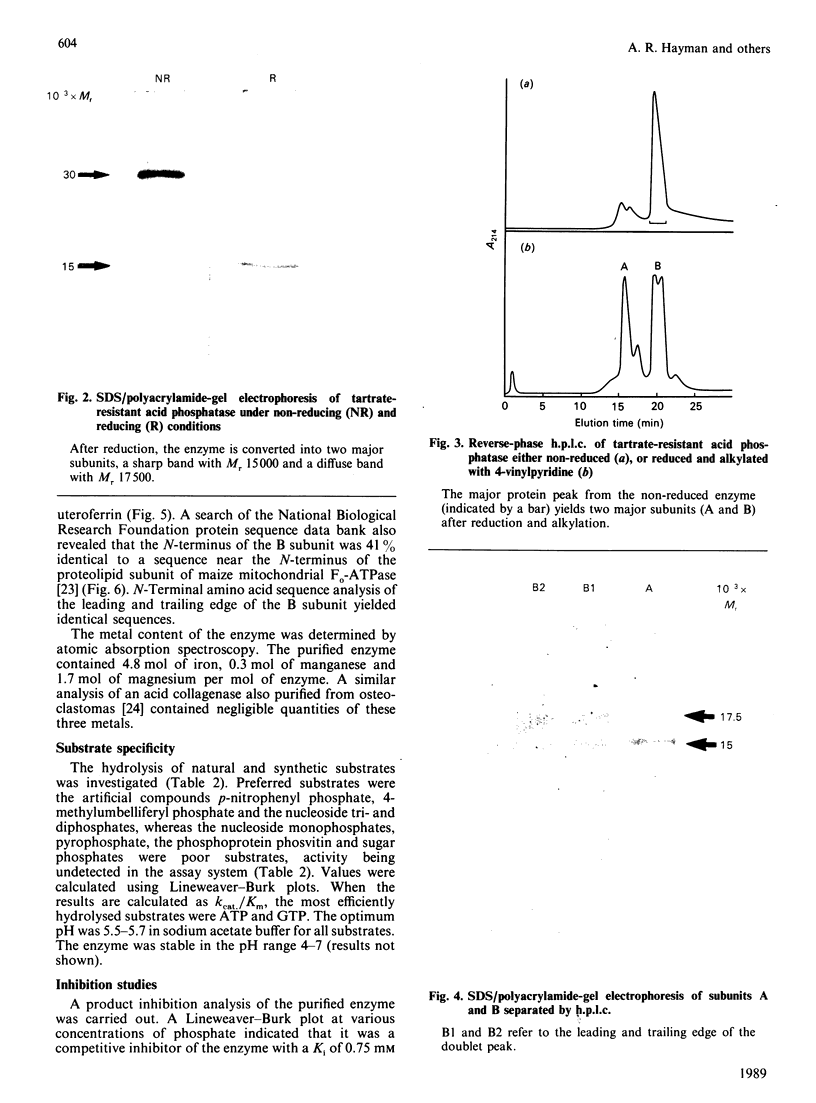
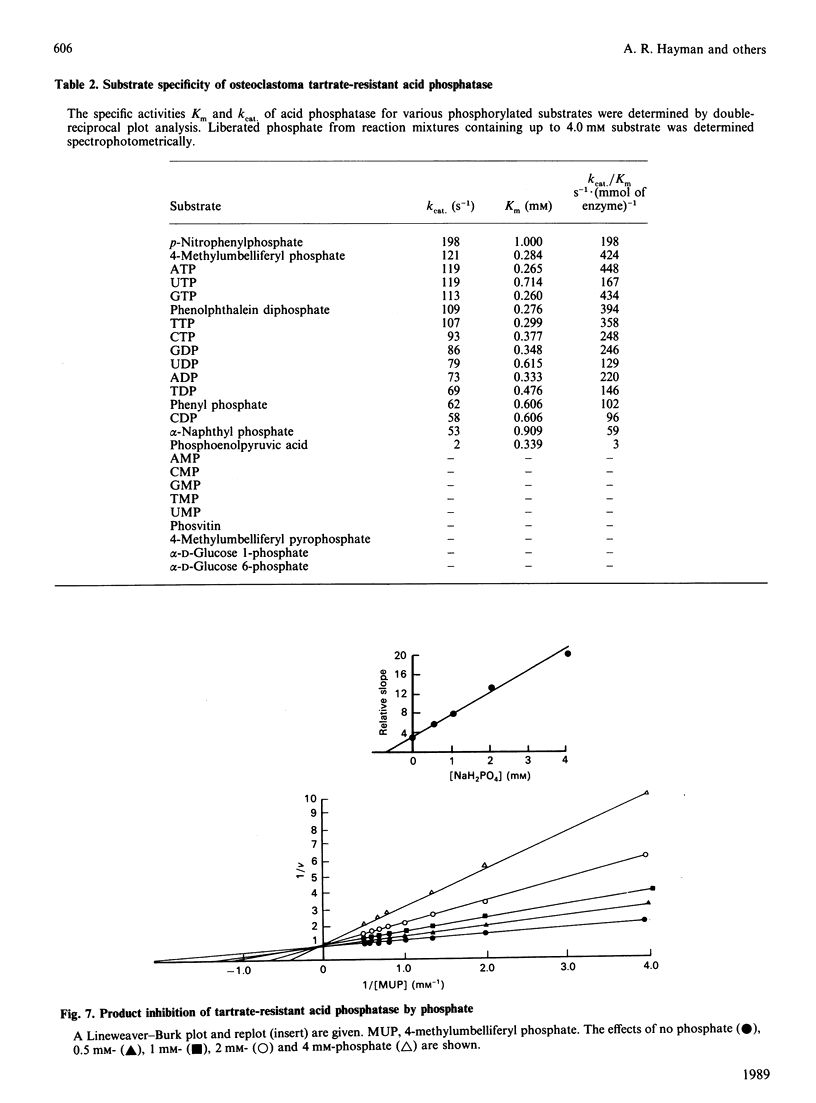
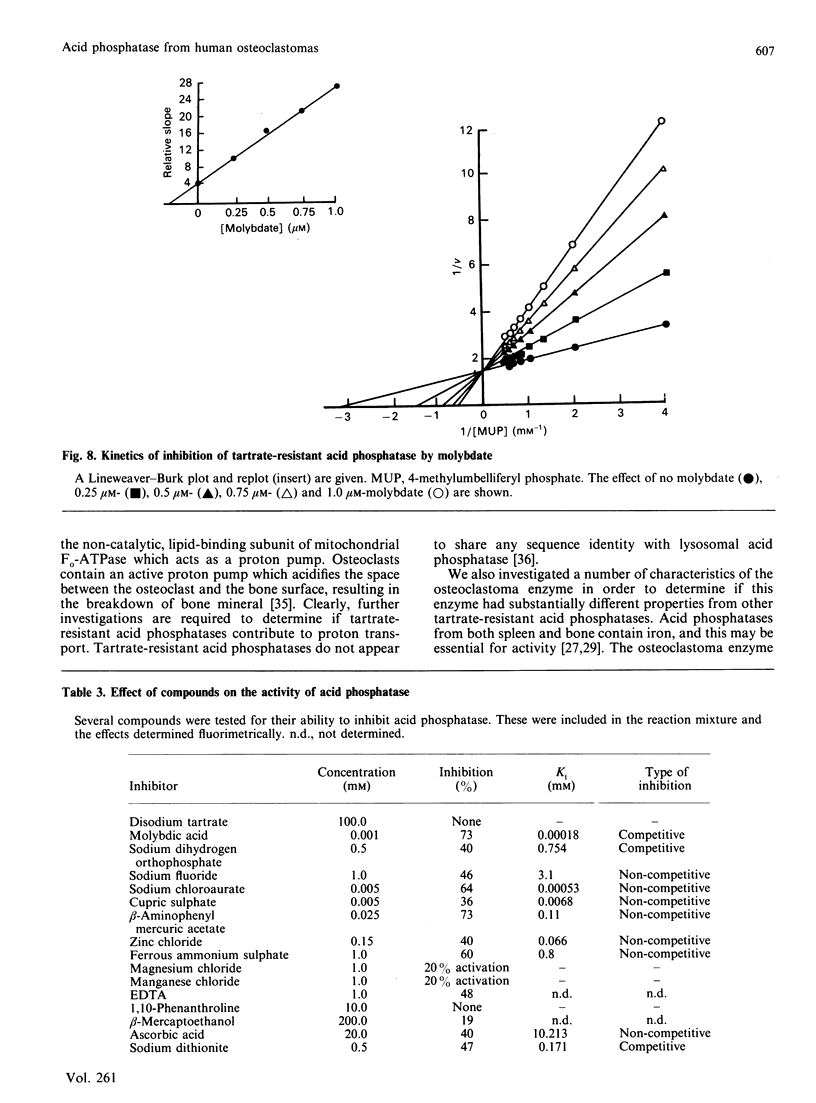
Tartrate-resistant acid phosphatase is one of the major enzymes produced and secreted by osteoclasts. To obtain sufficient enzyme for biochemical characterization, we have purified this enzyme from human osteoclastomas by sequential chromatography on SP-Sephadex, CM-Sephadex, hydroxylapatite, Sephadex G-150 and concanavalin A-Sepharose. The purification over the original tumour extract was about 2000-fold, with a yield of 10%. The enzyme appeared to be homogeneous when assessed by SDS/polyacrylamide-gel electrophoresis. Both gel filtration and SDS/polyacrylamide-gel electrophoresis indicated an Mr of about 30,000. The reduced and alkylated enzyme consists of two subunits with Mrs of 15,000 and 17,500. The N-terminal amino acid sequence of both subunits indicates that there is a high degree of identity between the osteoclastoma enzyme and similar enzymes purified from spleen and uterus. Using 4-methylumbelliferyl phosphate as substrate, the specific activity of the purified enzyme was 387 units.mg-1, and the Km was 284 microns. The pH optimum was 5.7. Unlike similar enzymes purified from human and bovine bone, osteoclastoma acid phosphatase is not activated by reducing agents (2-mercaptoethanol or ascorbic acid). The enzyme contains 4.8 mol of Fe2+/3+, 0.3 mol of Mn2+ and 1.7 mol of Mg2+ per mol of enzyme. Although the enzyme loses 50% of its activity in the presence of EDTA, it is not inhibited by the iron chelator 1,10-phenanthroline. However, the enzyme is activated to a small extent by Mn2+ and Mg2+. Using a variety of substrates and inhibitors, we demonstrate that there are differences between the osteoclastoma acid phosphatase and the enzyme purified from other sources.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T. R., Toverud S. U. Purification and partial characterization of two acid phosphatases from rat bone. Calcif Tissue Int. 1979 Jul 3;27(3):219–226. doi: 10.1007/BF02441189. [DOI] [PubMed] [Google Scholar]
- Andersson G., Ek-Rylander B., Hammarström L. Purification and characterization of a vanadate-sensitive nucleotide tri- and diphosphatase with acid pH optimum from rat bone. Arch Biochem Biophys. 1984 Feb 1;228(2):431–438. doi: 10.1016/0003-9861(84)90007-9. [DOI] [PubMed] [Google Scholar]
- Atkinson A., Gatenby A. D., Lowe A. G. The determination of inorganic orthophosphate in biological systems. Biochim Biophys Acta. 1973 Aug 17;320(1):195–204. doi: 10.1016/0304-4165(73)90178-5. [DOI] [PubMed] [Google Scholar]
- BURSTONE M. S. Histochemical demonstration of acid phosphatases with naphthol AS-phosphates. J Natl Cancer Inst. 1958 Sep;21(3):523–539. [PubMed] [Google Scholar]
- Baron R., Neff L., Louvard D., Courtoy P. J. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J Cell Biol. 1985 Dec;101(6):2210–2222. doi: 10.1083/jcb.101.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL D. M., MOSS D. W. Spectrofluorimetric determination of acid phosphatase activity. Clin Chim Acta. 1961 May;6:307–315. doi: 10.1016/0009-8981(61)90055-9. [DOI] [PubMed] [Google Scholar]
- Chambers J. P., Peters S. P., Glew R. H., Lee R. E., McCafferty L. R., Mercer D. W., Wenger D. A. Multiple forms of acid phosphatase activity in Gaucher's disease. Metabolism. 1978 Jul;27(7):801–814. doi: 10.1016/0026-0495(78)90215-9. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Fuller K., Darby J. A. Hormonal regulation of acid phosphatase release by osteoclasts disaggregated from neonatal rat bone. J Cell Physiol. 1987 Jul;132(1):90–96. doi: 10.1002/jcp.1041320112. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Fuller K., McSheehy P. M., Pringle J. A. The effects of calcium regulating hormones on bone resorption by isolated human osteoclastoma cells. J Pathol. 1985 Apr;145(4):297–305. doi: 10.1002/path.1711450403. [DOI] [PubMed] [Google Scholar]
- Chen J., Yam L. T., Janckila A. J., Li C. Y., Lam W. K. Significance of "high" acid phosphatase activity in the serum of normal children. Clin Chem. 1979 May;25(5):719–722. [PubMed] [Google Scholar]
- Davis J. C., Lin S. S., Averill B. A. Kinetics and optical spectroscopic studies on the purple acid phosphatase from beef spleen. Biochemistry. 1981 Jul 7;20(14):4062–4067. doi: 10.1021/bi00517a018. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Schuster A. M., Levings C. S., Timothy D. H. Nucleotide sequence of F(0)-ATPase proteolipid (subunit 9) gene of maize mitochondria. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1015–1019. doi: 10.1073/pnas.82.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis T., Moss D. W. Tartrate-resistant acid phosphatase of human lung: apparent identity with osteoclastic acid phosphatase. Enzyme. 1985;33(1):34–40. doi: 10.1159/000469401. [DOI] [PubMed] [Google Scholar]
- Eggert F. M. Stable acid phosphatase: II. Effects of pH and inhibitors. Histochemistry. 1980;66(3):319–329. doi: 10.1007/BF00495745. [DOI] [PubMed] [Google Scholar]
- Friedman M., Krull L. H., Cavins J. F. The chromatographic determination of cystine and cysteine residues in proteins as s-beta-(4-pyridylethyl)cysteine. J Biol Chem. 1970 Aug 10;245(15):3868–3871. [PubMed] [Google Scholar]
- Hefler S. K., Averill B. A. The "manganese(III)-containing" purple acid phosphatase from sweet potatoes is an iron enzyme. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1173–1177. doi: 10.1016/0006-291x(87)90771-6. [DOI] [PubMed] [Google Scholar]
- Helfrich M. H., Thesingh C. W., Mieremet R. H., van Iperen-van Gent A. S. Osteoclast generation from human fetal bone marrow in cocultures with murine fetal long bones. A model for in vitro study of human osteoclast formation and function. Cell Tissue Res. 1987 Jul;249(1):125–136. doi: 10.1007/BF00215426. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Lewis D., McNulty K., Pringle J. A., Chambers T. J. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 1985 Nov;45(11 Pt 2):5663–5669. [PubMed] [Google Scholar]
- Hunt D. F., Yates J. R., 3rd, Shabanowitz J., Zhu N. Z., Zirino T., Averill B. A., Daurat-Larroque S. T., Shewale J. G., Roberts R. M., Brew K. Sequence homology in the metalloproteins; purple acid phosphatase from beef spleen and uteroferrin from porcine uterus. Biochem Biophys Res Commun. 1987 May 14;144(3):1154–1160. doi: 10.1016/0006-291x(87)91432-x. [DOI] [PubMed] [Google Scholar]
- Kato T., Hara A., Nakayama T., Sawada H., Hamatake M., Matsumoto Y. Purification and characterization of purple acid phosphatase from rat bone. Comp Biochem Physiol B. 1986;83(4):813–817. doi: 10.1016/0305-0491(86)90152-5. [DOI] [PubMed] [Google Scholar]
- Ketcham C. M., Baumbach G. A., Bazer F. W., Roberts R. M. The type 5, acid phosphatase from spleen of humans with hairy cell leukemia. Purification, properties, immunological characterization, and comparison with porcine uteroferrin. J Biol Chem. 1985 May 10;260(9):5768–5776. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam K. W., Li C. Y., Yam L. T., Desnick R. J. Comparison of the tartrate-resistant acid phosphatase in Gaucher's disease and leukemic reticuloendotheliosis. Clin Biochem. 1981 Aug;14(4):177–181. doi: 10.1016/s0009-9120(81)91201-7. [DOI] [PubMed] [Google Scholar]
- Lam K. W., Li O., Li C. Y., Yam L. T. Biochemical properties of human prostatic acid phosphatase. Clin Chem. 1973 May;19(5):483–487. [PubMed] [Google Scholar]
- Lam K. W., Yam L. T. Biochemical characterization of the tartrate-resistant acid phosphatase of human spleen with leukemic reticuloendotheliosis as a pyrophosphatase. Clin Chem. 1977 Jan;23(1):89–94. [PubMed] [Google Scholar]
- Lau K. H., Freeman T. K., Baylink D. J. Purification and characterization of an acid phosphatase that displays phosphotyrosyl-protein phosphatase activity from bovine cortical bone matrix. J Biol Chem. 1987 Jan 25;262(3):1389–1397. [PubMed] [Google Scholar]
- Lau K. H., Onishi T., Wergedal J. E., Singer F. R., Baylink D. J. Characterization and assay of tartrate-resistant acid phosphatase activity in serum: potential use to assess bone resorption. Clin Chem. 1987 Apr;33(4):458–462. [PubMed] [Google Scholar]
- Pohlmann R., Krentler C., Schmidt B., Schröder W., Lorkowski G., Culley J., Mersmann G., Geier C., Waheed A., Gottschalk S. Human lysosomal acid phosphatase: cloning, expression and chromosomal assignment. EMBO J. 1988 Aug;7(8):2343–2350. doi: 10.1002/j.1460-2075.1988.tb03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. B., Glew R. H. A tartrate-resistant acid phosphatase from Gaucher spleen. Purification and properties. J Biol Chem. 1980 Jun 25;255(12):5864–5870. [PubMed] [Google Scholar]
- Robinson D. B., Glew R. H. Acid phosphatase in Gaucher's disease. Clin Chem. 1980 Mar;26(3):371–382. [PubMed] [Google Scholar]
- Schlosnagle D. C., Bazer F. W., Tsibris J. C., Roberts R. M. An iron-containing phosphatase induced by progesterone in the uterine fluids of pigs. J Biol Chem. 1974 Dec 10;249(23):7574–7579. [PubMed] [Google Scholar]
- Stepan J. J., Silinková-Málková E., Havránek T., Formánková J., Zichová M., Lachmanová J., Straková M., Broulik P., Pacovský V. Relationship of plasma tartrate resistant acid phosphatase to the bone isoenzyme of serum alkaline phosphatase in hyperparathyroidism. Clin Chim Acta. 1983 Sep 30;133(2):189–200. doi: 10.1016/0009-8981(83)90404-7. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Vaes G. On the mechanisms of bone resorption. The action of parathyroid hormone on the excretion and synthesis of lysosomal enzymes and on the extracellular release of acid by bone cells. J Cell Biol. 1968 Dec;39(3):676–697. doi: 10.1083/jcb.39.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wergedal J. E., Baylink D. J. Distribution of acid and alkaline phosphatase activity in undemineralized sections of the rat tibial diaphysis. J Histochem Cytochem. 1969 Dec;17(12):799–806. doi: 10.1177/17.12.799. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Finkel H. E. Leukemic reticuloendotheliosis. The role of tartrate-resistant acid phosphatase in diagnosis and splenectomy in treatment. Arch Intern Med. 1972 Aug;130(2):248–256. doi: 10.1001/archinte.130.2.248. [DOI] [PubMed] [Google Scholar]