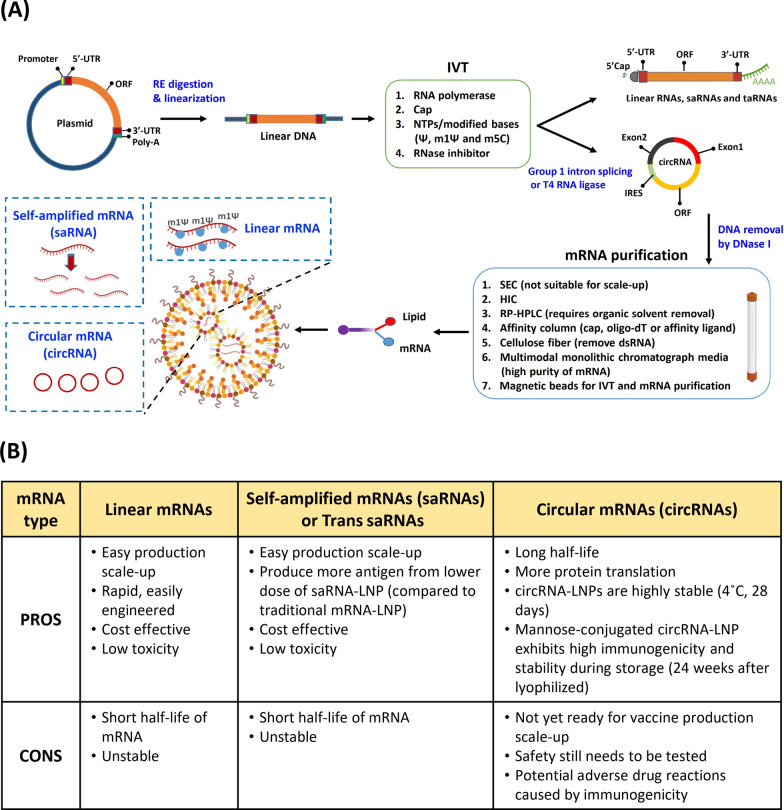
Fig. 2.
Synthesis and purification of three distinct mRNA types for mRNA-LNP drugs. A The in vitro transcription (IVT) process is illustrated. First, a plasmid is generated containing the target gene with a T7 promoter. After restriction enzyme (RE) digestion of the plasmid and purification of linear DNA, IVT is performed with T7 RNA polymerase, cap analogue, modified bases, and RNase inhibitor to generate transcribed linear mRNAs. The linear mRNAs may be traditional linear mRNAs, self-amplified mRNAs (saRNAs), or trans-amplified mRNAs (taRNAs). For production of circular RNAs (circRNAs), cyclization is achieved via intron-splicing reaction or T4 RNA ligase. Impurities within the mRNA products may be eliminated by DNA digestion and mRNA purification, along with other methods specific to the type of RNA product. The highly purified mRNAs are suitable for incorporation into mRNA-LNP formulations. (SEC: size exclusion chromatography, HIC: hydrophobic interaction chromatography, RP-HPLC: reverse phase HPLC). B Advantages and disadvantages of the three different mRNA types used for mRNA-LNP drugs are shown