Abstract
An important but unresolved question is whether mammalian mitochondria metabolize arginine to agmatine by the ADC (arginine decarboxylase) reaction. 15N-labelled arginine was used as a precursor to address this question and to determine the flux through the ADC reaction in isolated mitochondria obtained from rat liver. In addition, liver perfusion system was used to examine a possible action of insulin, glucagon or cAMP on a flux through the ADC reaction. In mitochondria and liver perfusion, 15N-labelled agmatine was generated from external 15N-labelled arginine. The production of 15N-labelled agmatine was time- and dose-dependent. The time-course of [U-15N4]agmatine formation from 2 mM [U-15N4]arginine was best fitted to a one-phase exponential curve with a production rate of approx. 29 pmol·min−1·(mg of protein)−1. Experiments with an increasing concentration (0– 40 mM) of [guanidino-15N2]arginine showed a Michaelis constant Km for arginine of 46 mM and a Vmax of 3.7 nmol·min−1·(mg of protein)−1 for flux through the ADC reaction. Experiments with broken mitochondria showed little changes in Vmax or Km values, suggesting that mitochondrial arginine uptake had little effect on the observed Vmax or Km values. Experiments with liver perfusion demonstrated that over 95% of the effluent agmatine was derived from perfusate [guanidino-15N2]arginine regardless of the experimental condition. However, the output of 15N-labelled agmatine (nmol·min−1·g−1) increased by approx. 2-fold (P<0.05) in perfusions with cAMP. The findings of the present study provide compelling evidence that mitochondrial ADC is present in the rat liver, and suggest that cAMP may stimulate flux through this pathway.
Keywords: arginase, arginine decarboxylase, cAMP, glucagon, insulin, ornithine
Abbreviations: ADC, arginine decarboxylase; HFAA, hexafluoroacetylacetone; PKA, protein kinase A
INTRODUCTION
An important but unresolved question is whether agmatine is synthesized in mammalian mitochondria by the ADC (arginine decarboxylase) reaction. Agmatine, which is widely distributed in mammalian tissue [1–4], may have a role as a hormone and/or polyamine precursor for multiple metabolic functions. Numerous studies have shown that agmatine affects a number of physiological processes, including the regulation of intracellular polyamine levels and cellular proliferation, regulation of neurotransmitter receptors, inhibition of NO synthesis, release of insulin from β-cells and regulation of body nitrogen economy secondary to its action on hepatic ureagenesis [5–10]. Therefore it is important to determine whether agmatine is formed in mammalian tissue or exclusively derived from food intake and/or intestinal flora.
The presence of the ADC reaction has been well established in bacteria and plants [11,12]. A similar reaction in mammalian mitochondria was first identified in 1994 [5]. Since then, it has been shown that agmatine is present in several mammalian tissues and that the ADC reaction is active in various tissues and organs [2,3,5,6,10]. In addition, ADC has been partially cloned in the rat kidney [13], and a human cDNA clone exhibiting ADC activity has been described in [14]. Notwithstanding these reports demonstrating the presence of the ADC reaction in mammalian tissues, Pegg and co-workers [15] detected no 14C-labelled agmatine after incubation of mouse or rat mitochondrial extract with [U-14C]arginine. These authors argued that earlier studies demonstrating ADC activity were erroneous, and that the apparent presence of agmatine in mammalian tissue may correspond to bacterial contamination, food intake and/or intestinal flora [15].
A major impediment in the study of ADC activity and/or measurement of [agmatine] has been the lack of a precise and specific tool for determining agmatine formation. The use of 14CO2 release from 14C-labelled arginine cannot differentiate between 14CO2 released by the ADC reaction, the ornithine decarboxylase reaction, or mitochondrial oxidation of ornithine. Furthermore, the use of HPLC for determination of [agmatine] is somewhat problematic due to the low tissue [agmatine] and its poor separation from other amino acids present in the tissue [1,4]. The reported concentration of agmatine as determined by HPLC has ranged between pmol/g wet weight to μmol/g wet weight [1–4,10]. However, the use of 15N and GC–MS methodology allows determination of the true product of the ADC reaction as well as the precise tissue agmatine level. With 15N-labelled arginine as a precursor and GC–MS as an analytical tool, one can precisely and specifically determine the production of 15N-labelled agmatine by the ADC reaction. In addition, the isotope dilution approach allows for an accurate determination of [agmatine] in the picomolar range.
In the present study, we took advantage of 15N and GC–MS methodology to characterize the kinetic parameters of ADC in isolated hepatic mitochondria of overnight fasted rats. In addition, a liver perfusion system was used to examine a possible action of insulin, glucagon or cAMP on flux through the ADC reaction. The results demonstrate that 15N-labelled agmatine was formed in experiments with isolated mitochondria or during liver perfusions with 15N-labelled arginine. The results suggest that cAMP may stimulate flux through the ADC reaction.
MATERIALS AND METHODS
Experiments with isolated mitochondria
Mitochondria were isolated from the liver of overnight fasted rats by differential centrifugation as described previously [16]. Briefly, the liver of an anaesthetized rat was cannulated through the portal vein and rinsed with 0.9% NaCl solution (4 °C), excised and weighed. The minced liver was homogenized in a glass Potter–Elvehjem homogenizer with a Teflon® pestle in 12.5 volumes of cooled (4 °C) isolation buffer consisting of (mM) mannitol (225), sucrose (75), EGTA (1) and Hepes (5), pH 7.4. The mitochondrial pellet was gently resuspended with isolation medium to yield 50–80 mg of protein/ml. All manipulations were performed in a cold room and the mitochondrial suspension was kept on ice.
The basic incubation medium consisted of (mM) Tris (50), KCl (5), MgCl2 (5), KHCO3 (15), KH2PO4 (5), α-ketoglutarate (1), succinate (5), ATP (5), octanoic acid (2) and EDTA (2), pH 7.4. Mitochondrial suspensions (2 ml; ∼3 mg of mitochondrial protein/ml) were incubated at 30 °C for the times indicated and with the addition of 15N-labelled arginine and other modulators. In the first series of experiments, we sought to determine the time-course of agmatine production from [U-15N4]arginine. The level of arginine in mitochondria isolated from overnight fasted rats was 1.34±0.39 nmol/mg of protein (mean±S.D.; n=13 mitochondrial preparations from separate rats during a period of six months). Assuming a matrix volume of 1 μl/mg of protein [17], mitochondrial [arginine] is approx. 1.34±0.39 mM. Therefore whole mitochondria were incubated with 2 mM [U-15N4]arginine, with or without 2 mM unlabelled ornithine, to determine a possible effect of cytosolic ornithine on mitochondrial production of agmatine, as previously indicated in [2].
To determine the Michaelis constant Km for arginine and the Vmax of the flux through the ADC reaction, we used the initial linear stage of 15N-labelled agmatine formation obtained in time-course experiments, indicated above. To this end, mitochondria were incubated for 7 min at 30 °C, with an increasing concentration (0– 40 mM) of [guanidino-15N2]arginine. The production of [guanidino-15N2]agmatine was used to determine the Km and the Vmax values.
The above time-course incubations and experiments with an increasing concentration of [guanidino-15N2]arginine were performed in the presence of 2 mM EDTA. However, it has been shown that free Ca2+ inhibits the ADC reaction [2]. Furthermore, arginine is a substrate for both ADC and arginase (arginase-I and/or arginase-II) [18,19]. Arginase may compete with ADC for arginine, and thus diminish the production of agmatine. It has been demonstrated that Mn2+ stimulates the activity of arginases (arginase-I and -II) [20]. Therefore to determine a possible effect of the mitochondrial bound arginase or mitochondrial free Ca2+ on the flux through the ADC reaction, an additional series of experiments was performed with 0.1 mM Mn2+ without EDTA. The addition of Mn2+ is expected to stimulate the arginase reaction and the omission of EDTA to preserve mitochondrial free [Ca2+].
From each of the incubations outlined above, an aliquot (100 μl) was taken for protein determination and the incubation was stopped with 100–150 μl of HClO4 (60%). The deproteinized extracts were neutralized and assayed for agmatine.
Experiments with broken mitochondria
The measured Km for arginine in experiments with intact mitochondria may be affected by mitochondrial uptake of arginine. To address this possibility, experiments with an increasing concentration of [guanidino-15N2]arginine were repeated with broken mitochondria. Because mitochondrial matrix is required for ADC activity [2] experiments with broken mitochondria will preserve mitochondrial matrix and ADC activity. Broken mitochondria were prepared from stock of intact mitochondrial pellet, subjected to three cycles of freezing in liquid nitrogen and thawing as described in [21]. The resulting mixture of matrix and mitochondrial membranes was suspended with a basic incubation medium (as indicated above), at a protein concentration of approx. 4 mg/ml. Then 2 ml of the suspension containing the matrix and mitochondrial membranes was incubated with an increasing concentration of [guanidino-15N2]arginine similar to the experiments with whole mitochondria.
Measurement of mitochondrial respiration
Oxygen consumption in experiments with whole mitochondria was measured at 26 °C using a Clark electrode and the Oxygen Measuring System (Instech SYS203, Plymouth Meeting, PA, U.S.A.). Respiratory control was determined in each batch of mitochondrial preparation according to Chance and Williams [22]. An aliquot of mitochondrial preparation was added to a polarographic cell containing basic incubation medium with only 5 mM succinate and 1 mM α-ketoglutarate. The respiration rate at state two (V2) was recorded when O2 consumption became linear. Then 0.3 mM ADP was added to establish the respiration rate at state three (V3). When most of ADP was converted to ATP, respiration rate at state four (V4) was recorded. In most cases, oxygen consumption was 2–3, 9–12 and 2–3 nmol of O2·min−1·(mg of protein)−1 for state two, three and four respectively, and V3/V2 or V3/V4 ratio was between 3 and 4. All experiments were performed with whole mitochondria having a V3/V2 ratio greater than 3. However, broken mitochondria had minimal or no oxygen consumption.
Experiments with liver perfusions
Livers from overnight fasted male rats were perfused in the non-recirculating mode as previously described in [10,23]. Briefly, the basic perfusion medium was Krebs saline continuously gassed with 95% O2/5% CO2 and containing lactate (2.1 mM) and pyruvate (0.3 mM) as metabolic fuels. The flow rate (3–3.5 ml·min−1·g−1), pH and pO2 (in influent and effluent media) were monitored throughout, and oxygen consumption was calculated. After 20 min of preperfusion, the basic perfusate was replaced by perfusate that contained, in addition to the lactate and pyruvate, precursors for urea nitrogen (i.e., 0.3 mM NH4Cl and 1 mM glutamine), and 0.5 mM L-[guanidino-15N2]arginine in the absence (control) or presence of 10−7 M insulin, glucagon or 10−4 M dibutyryl-cAMP, a permeable cAMP analogue. Samples were taken from the influent and effluent media for chemical and 15N-GC–MS analyses. At the end of the perfusion, the liver was freeze-clamped, treated with HClO4, and metabolite measurements were done in neutralized extracts as indicated [10,23].
To determine the baseline concentration of hepatic agmatine, livers from a separate group of overnight fasted rats were rinsed for 5 min with cold (4 °C) saline, after which livers were freeze-clamped, treated with HClO4 and [agmatine] was determined by spiking the extract (300 μl) with [guanidino-15N2]agmatine. The latter was prepared as described previously [10]. Determination of [agmatine] after liver perfusion with L-[guanidino-15N2]arginine was accomplished as described below.
GC–MS methodology and determination of 15N-labelled agmatine
Isotopic enrichment in 15N-labelled agmatine was measured with GC–MS using a modification of a method described previously [24]. To an aliquot of ∼300 μl of mitochondrial extract, 150 μl of 4 M NH4OH was added. Thereafter, the sample was loaded into an acetate 1-X8 Dowex resin column and agmatine was eluted with 3– 4 ml of H2O and dried down under a stream of N2 gas. Then 50 μl of HFAA (hexafluoroacetylacetone) was added to vials containing the dry sample, vortex-mixed and heated at 110 °C for 60 min. The reaction mixture was cooled to room temperature (∼22–25 °C), dried under a stream of N2 gas and dissolved with 1 ml of 3 M HCl. The HFAA derivative of agmatine was extracted with 1 ml of ethyl acetate. The extract was dried under a stream of N2 gas and dissolved in 75 μl of ethyl acetate. Approximately 2–4 μl of ethyl acetate extract of the HFAA-agmatine derivative was injected into the GC–MS system for analysis.
Samples were analysed by either a GC–MS Agilent System (6890 GC-5973 Mass Selective Detector) or a Hewlett-Packard MSD (HP-5970). MS was performed using electron impact (EI) ionization with an ionizing voltage of −70 eV and an electron multiplier set to 2000 V. The GC injector temperature was set at 250 °C and transfer line at 280 °C. The usual GC temperature program was: 80 °C for 2 min, and then 30 °C/min up to 300 °C. The capillary column was a SUPELCOWAX™-10, 15 m×0.32 mm×0.25 μl film thickness. 15N enrichment in the HFAA derivative of [guanidino-15N2]agmatine was monitored using the m/z ratio 286/284 and, for [U-15N4]agmatine, using the m/z ratio 287/284.
Samples obtained from experiments with liver perfusions or mitochondrial incubations were analysed for [agmatine] by isotope dilution as follows. First, the initial (I1) isotopic enrichment in 15N-labelled agmatine was determined. Secondly, a parallel set of the same samples was spiked with a known amount of unlabelled agmatine (d), and a second measurement (I2) of 15N-labelled agmatine was taken. [Agmatine] was calculated as described in [25]. Amino acids were determined with HPLC using precolumn derivatization with o-phthalaldehyde [26].
Calculations and data analyses
Data obtained from mitochondrial incubation were analysed with GraphPad Prism-4 software for linear and nonlinear curve fitting. The production of [guanidino-15N4]agmatine during the course of incubations with L-[U-15N4]arginine was best fitted to a one-phase exponential association [Y=Ymax*(1–e(−kt))], and the flux through the ADC reaction [nmol·min−1·(mg of protein)−1] was given by the product of Ymax*k. The GraphPad Prism-4 software was also used in experiments with an increasing concentration of arginine to determine the best curve fit and to calculate the Vmax for the flux through the ADC reaction and the Km for arginine.
During liver perfusions, the rate of agmatine output was determined by the measurement of agmatine concentration in the effluent (nmol/ml), normalized to the flow rate (ml/min) and liver wet weight, as described previously [10,22]. The output of [guanidino-15N2]agmatine was calculated from the product of 15N enrichment, at.% excess/100 times concentration [nmol·min−1·(g wet weight)−1] and is expressed (nmol of [guanidino-15N2]-agmatine)·min−1·(g wet weight)−1. Flux through the ADC reaction is represented by the output in the effluent of [guanidino-15N2]agmatine during the course of perfusion with L-[guanidino-15N2]arginine.
Each series of experiments with isolated mitochondria was repeated 4–6 times, and 3–4 times with liver perfusion, as outlined above. Statistical analysis was performed using In-STAT 1.14 software for the Macintosh. Student's t test or ANOVA was employed to compare two groups or differences among groups as needed. P<0.05 was taken as indicating a statistically significant difference.
RESULTS
Measurement of agmatine: analytical consideration
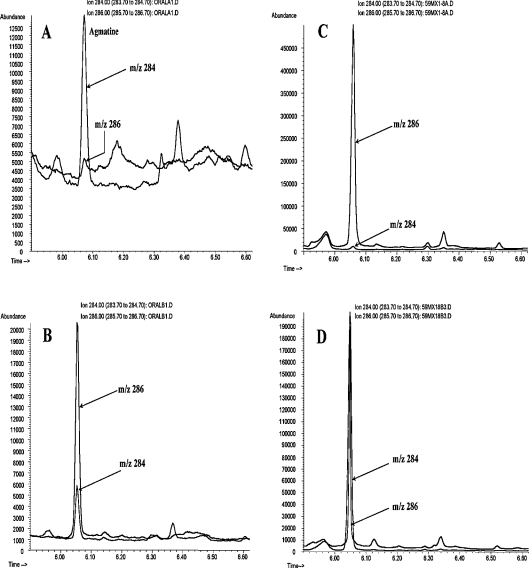
Figure 1 illustrates a typical GC–MS chromatogram of the HFAA-agmatine derivative obtained from mitochondrial incubation or freeze-clamped liver extract. This representative chromatogram demonstrates an excellent separation of agmatine from other tissue constituents. There is a high signal-to-noise ratio for the ions at m/z 286 and 284 even with as little as 2 pmol of agmatine. The chromatogram in Figure 1(A) corresponds to the injection of 2–3 μl of the HFAA-derivative containing approx. 2–4 pmol agmatine.
Figure 1. A representative GC–MS chromatogram of single ion monitoring of the HFAA derivative of agmatine.
(A) A freeze-clamped liver extract from an overnight fasted rat. The chromatogram corresponds to approx. 2 pmol of agmatine (m/z 284). (B) Same extract shown in (A) after spiking with [guanidino-15N2]agmatine (2 nmol/g). (C) Mitochondrial extract after incubation for 7 min with 10 mM [guanidino-15N2]arginine. Peak corresponds to approx. 4 pmol of [guanidino-15N2]agmatine (m/z 286). (D) Same extract shown in (C) after the addition of unlabelled agmatine (∼1.2 nmol/3 mg of protein). The m/z ratio 286/284 was used to determine the isotopic enrichment as well as the concentration of agmatine by isotope dilution.
The EI ionization of the HFAA-agmatine derivative shows a major ion at m/z 284 for unlabelled agmatine. This ion contains three N due to the cleavage of one N (the original α-N of arginine) [25]. Thus [guanidino-15N2]agmatine and [U-15N4]agmatine are monitored at m/z 286 and 287 respectively. Multiple measurements indicated that the natural abundance of m/z 286/284 and 287/284 ratios is 6 and 0.9% respectively. This relatively low natural background value allows for precise measurement of 15N enrichment (<1 at.% excess) in agmatine.
Production of agmatine in isolated mitochondria
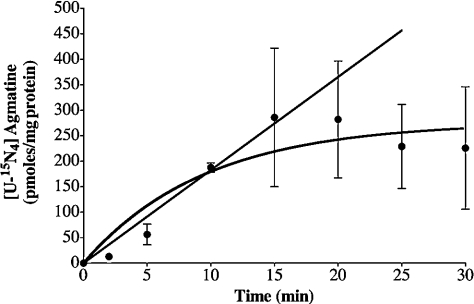
To determine whether ADC activity exists in mitochondria isolated from rat liver, an initial series of experiments was designed to determine the time-course of 15N-labelled agmatine production during incubations with a physiological level of mitochondrial arginine. Figure 2 illustrates the time-course of [U-15N4]agmatine production, which was best fitted to a one-phase exponential curve (r2=0.87). Production of 15N-labelled agmatine increased almost linearly up to 15 min and then reached a plateau between 15 and 30 min. Data points presented in this curve were used to calculate the rate constant (k) and flux through the ADC reaction. The means±S.E.M. for these parameters are: 0.11±0.05 min−1 and 29±5.1 (pmol·min−1·mg−1) for k and flux through the ADC reaction respectively. Inclusion of 2 mM ornithine had little effect on the time-course of [U-15N4]agmatine production.
Figure 2. Time-course of 15N-labelled agmatine production during the incubation of whole mitochondria with [U-15N4]arginine.
Experiments were performed with [U-15N4]arginine (2 mM) and other metabolites as indicated under the Materials and methods section. The line shows the best fit (r2=0.87) of a single-phase exponential association [Y=Ymax*(1−e(−kt))]. The straight line corresponds to the linear stage of the reaction (r2=0.97). 15N-labelled agmatine was determined by the product of 15N enrichment (at.% excess/100) times total amount (pmol/mg of protein). Results are expressed as means±S.D. for 4–6 independent experiments.
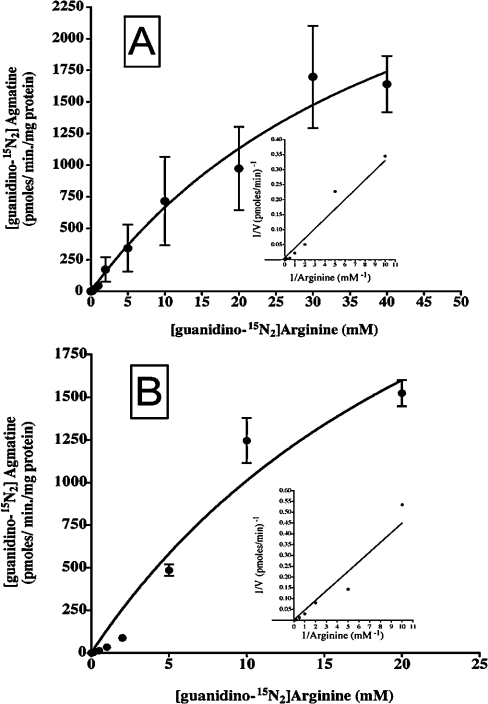
The next series of experiments was designed to characterize the Km for arginine and Vmax of the ADC reaction. Isolated mitochondria were incubated for 7 min with an increasing concentration of [guanidino-15N2]arginine (Figure 3A). Half-maximal arginine decarboxylation (the Km for arginine) was achieved at 46±18.8 mM with a Vmax of 3.74±0.94 nmol·min−1·(mg of protein)−1. The presence or absence of EDTA and/or Mn2+ had little effect on these kinetic parameters. In experiments with broken mitochondria, the Vmax and Km for arginine were 3.8±1.7 nmol·min−1·(mg of protein)−1 and 28.2±17.8 mM respectively (Figure 3B). Therefore arginine transport into mitochondria is not rate limiting in the production of agmatine, and may have little or no effect on the observed kinetic parameters of ADC in experiments with whole mitochondria.
Figure 3. Saturation curves of 15N-labelled agmatine production from [guanidino-15N2]arginine.
(A) Experiments with intact mitochondria and (B) experiments with broken mitochondria. Insets represent a Lineweaver–Burk plot of these data. Results were obtained after 7 min incubation of whole or broken mitochondria with increasing [arginine] as indicated. Results are the means (n=3–5) for the combined results obtained from experiments with the addition of Mn2+ (0.1 mM) and no EDTA or with EDTA (2 mM) and without the addition of Mn2+. The lines represent the best fit (r2>0.90). Results are expressed as means±S.D.
Hormonal regulation of the ADC reaction in liver perfusion system
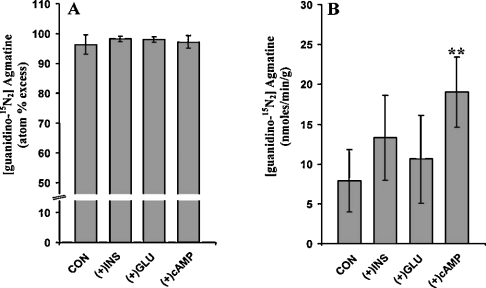
After the demonstration that agmatine is formed from arginine in mitochondria isolated from rat liver, experiments with the liver perfusion system were performed to determine the action of insulin, glucagon or dibutyryl-cAMP on the flux through the ADC reaction. Results in Figure 4 (panel A) demonstrate that over 95% of the effluent agmatine was derived from perfusate [guanidino-15N2]arginine, regardless of the experimental conditions. However, the output of 15N-labelled agmatine (Figure 4, panel B) increased by approx. 30% (P>0.05) in perfusions with glucagon or insulin, i.e., 7.9±3.9, 10.6±5.5 and 13.3±5.3 nmol·min−1·g−1 in control, glucagon or insulin respectively (mean±S.D.), and significantly (P<0.05) increased in perfusions with cAMP (19.03±4.4 nmol·min−1·g−1). These observations suggest that insulin and glucagon may have insignificant effect, whereas cAMP may stimulate the flux through the ADC reaction.
Figure 4. Production and output of 15N-labelled agmatine from livers perfused with [guanidino-15N2]arginine.
(A) 15N enrichment (at.% excess) in effluent agmatine. (B) Output (nmol·min−1·g−1) of 15N-labelled agmatine as determined by the product of 15N enrichment (at.% excess/100) times total output (nmol·min−1·g−1). Data were obtained from measurements done at the steady-state (i.e. 25–40 min after start of 0.5 mM [guanidino-15N2]arginine infusion and other nutrients as indicated under the Materials and methods section without (CON, control) or with the addition of 10−7 M insulin (+INS), glucagon (+GLU) or 10−4 M dibutyryl-cAMP. Results are expressed as means±S.D. for three livers. **P<0.05.
Measurement of 15N enrichment in [guanidino-15N2]agmatine in freeze-clamped livers at the end of perfusions with [guanidino-15N2]arginine demonstrates that approx. 46, 54, 56 and 78% of agmatine in the liver was in the form of [guanidino-15N2]agmatine, in control, perfusions with glucagon, insulin or dibutyryl-cAMP respectively. Furthermore, the baseline agmatine content in freeze-clamped liver extracts obtained from overnight fasted rats (without perfusion) was 1.2±0.6 nmol/g (n=3). This value increased to 6.1±2.2, 9.1±3.7, 3.1±1.9 and 8.2±0.9 nmol/g in livers perfused with 15N-labelled arginine without hormone (n=4), in perfusion with insulin (n=3), with dibutyryl-cAMP (n=3) or in perfusions with glucagon (n=3) respectively. These observations illustrate that hepatic [agmatine] increased by approx. 4-fold after perfusion with 0.5 mM arginine. The apparent differences between control and perfusions with dibutyryl-cAMP, insulin or glucagon are statistically insignificant. The formation of 15N-labelled agmatine during liver perfusions and the increased [agmatine] in liver extract must have occurred after decarboxylation of the perfusate 15N-labelled arginine. These findings, together with the above results obtained from isolated mitochondria, are in agreement with earlier studies demonstrating the presence of the ADC reaction in mammalian tissue [2,3,5,6].
DISCUSSION
The mitochondrial enzyme ADC (EC 4.1.1.19) has long been known to be present in bacteria, invertebrates and plants [11,12,27]. ADC activity was first recognized in mammalian tissue in 1994 [5]. Thereafter, the activity of ADC has been demonstrated in a number of tissues and organs [2,3,5,9,10]. The findings of the present study (Figures 1–4) are in accordance with these earlier studies and provide compelling evidence that mitochondrial ADC is present in rat liver. Several observations presented here bear directly on this conclusion.
Mitochondrial incubations with either [U-15N4]- or [guanidino-15N2]-labelled arginine demonstrate: (i) the time-course of 15N-labelled agmatine production (Figure 2); and (ii) the apparent Michaelis–Menten behaviour of 15N-labelled agmatine production with increasing [arginine] (Figure 3). In the liver perfusion system the results demonstrate: (i) approximately 95–100% of the effluent agmatine was in the form of [guanidino-15N2] (Figure 4), indicating that the perfusate [guanidino-15N2]arginine was the exclusive source of the effluent agmatine; (ii) approximately, 46, 54, 56 and 78% of agmatine in the liver extracts was in the form of [guanidino-15N2]agmatine, in control, perfusions with glucagon, insulin and dibutyryl-cAMP respectively. The formation of [guanidino-15N2]agmatine can occur only after the decarboxylation of [guanidino-15N2]arginine by the ADC reaction; and (iii) agmatine content in liver extracts at the end of perfusion with [guanidino-15N2]arginine was approx. 3–4-fold higher than the baseline level.
The analyses of the present study were performed with the use of 15N and GC–MS methodology. This approach provides an excellent separation, identification and accurate quantification of agmatine in a picomolar range (Figure 1). Analysis of agmatine with HPLC did not provide a complete separation of agmatine from other tissue constituents. In most cases, the peak of the o-phthaldialdehyde derivative of tryptophan overlapped the peak of agmatine (results not shown), resulting in an overestimation of tissue [agmatine]. Purification of the tissue as previously indicated in [1,4] resulted in a poor yield and an underestimation of tissue [agmatine].
In experiments with physiological [arginine] and whole mitochondria, the production of 15N-labelled agmatine was linear between 0 and 15 min (Figure 2). The addition of 2 mM ornithine had little effect on flux through the ADC reaction. Similarly, the omission of EDTA when Mn2+ was added to the incubation had little effect on the Km for arginine or the Vmax of ADC. Incubations with Mn2+ significantly stimulated the production of urea by the mitochondrial bound arginase (results not shown), with only little effect on the flux through the ADC reaction. However, the observations demonstrated that 70–80% of arginine added to the incubation system was catabolized by arginase bound to the outer mitochondrial membrane, even without the addition of Mn2+. Therefore the high value of Km for arginine may be due to high catabolism of arginine by arginase bound to the outer mitochondrial membrane, thereby diminishing the availability of arginine for the intramitochondrial ADC reaction. The high Km for arginine (15-fold greater than physiological [arginine]) cannot reflect arginine transport into mitochondria because the Km was similar whether studies were performed with whole or disrupted mitochondria. Further evidence supporting this conclusion is provided by the lack of effect on the kinetic parameters of ADC in experiments with the addition of ornithine, which may inhibit mitochondrial arginine uptake [28,29]. Furthermore, a high-affinity mitochondrial transporter for arginine was found with a Km of 0.08 mM and Vmax of 1.89 nmol·min−1·(mg of protein)−1 [28], indicating robust mitochondrial arginine uptake compared with the apparent low velocity of the ADC reaction. The present study suggests a low affinity of ADC for arginine, which suggests relatively low flux even when [arginine] is 15–20-fold higher than the physiological level. Therefore, with physiological [arginine], the production of agmatine may be negligible compared with the dietary intake of agmatine.
The findings of the present study somewhat differ from those previously reported in [2]. Using mitochondrial membranes obtained from the rat brain, Regunathan and Reis [2] found that the ADC reaction was linear up to 90 min with an initial Km of 0.75 mM and Vmax of 2.22 nmol·h−1·(mg of protein)−1. A possible explanation for these differences is that in the previous study [2], the kinetic parameters were determined by the formation of 14CO2 from [1-14C]arginine. This approach is less specific for measurement of the ADC activity compared with the 15N-GC–MS technique employed in the present study. This is especially true when taking into account the observation showing that the ADC reaction also produced 14CO2 from [1-14C]ornithine [2]. In addition, a significant portion of [1-14C]arginine probably metabolized by mitochondrial bound arginase to form [1-14C]ornithine. The latter may be oxidized to form 14CO2. The sum of 14CO2 generated from [1-14C]arginine and [1-14C]ornithine may lead to a low Km value for arginine and overestimation of the Vmax for the ADC reaction. Thus a study of ADC kinetic parameters based on 14CO2 production may not provide specific and accurate kinetic parameters for the ADC reaction. However, the use of 15N-labelled arginine as substrate and measurement of 15N-labelled agmatine would provide an accurate relationship between the [substrate] and [product], and thereby reliable values of Km for arginine and Vmax for the ADC pathway.
In addition, Regunathan and Reis [2] indicated that ADC was inhibited by ornithine or Ca2+. In the present study, the omission of EDTA or the presence of 2 mM ornithine had no significant effect on the observed values of Km or Vmax. However, an explanation for the apparent differences between the observations of the present study and previous results is not feasible because: (a) in the earlier study, the effect of Ca2+ was evaluated with the addition of superphysiological [Ca2+] in experiments with mitochondrial membrane preparations. The present study was performed using whole mitochondria without supplementation of external Ca2+. Most of the mitochondrial [Ca2+] is in the form of a phosphate salt. Thus the omission or addition of EDTA from the incubation medium may have little effect on the mitochondrial Ca2+ pool when mitochondria were isolated with a medium containing 1 mM EGTA. (b) In the present study, 2 mM ornithine was added to the incubation medium in experiments with whole mitochondria. However, in a previous study [2], aside from the statement that ornithine inhibited ADC activity, there is no information regarding the [ornithine] used, the IC50 for ornithine and the nature of this inhibition.
Similarly, observations of the present study are not in agreement with a recent study by Pegg and co-workers [15], who found only a release of 14CO2 but failed to detect [U-14C]agmatine after incubation of mitochondrial extract with L-[U-14C]arginine. It was speculated that 14CO2 was strictly generated by oxidation of [U-14C]ornithine produced by the arginase reaction. Although our experiments with [U-15N4]arginine demonstrated that 15N-labelled urea and ornithine were the primary products of mitochondrial arginine catabolism (results not shown), the same experiments also documented the production of [U-15N4]agmatine (Figures 2 and 3). As indicated above, the affinity of ADC for arginine is very low. Therefore it is possible that in the earlier study, the [arginine] used (not reported) was too low, and thus the production of [U-14C]agmatine was below the detection limit of the method used. The finding that “14CO2 was produced in substantial amounts” [15], is expected because CO2 may be formed by ornithine decarboxylase [32], the tricarboxylic acid cycle [30,31] and the ADC reaction [2]. The substantial generation of 14CO2 would represent CO2 generated through all these pathways, including the ADC reaction. Again, as indicated above, studies of ADC activity based on measurements of 14CO2 generation may provide erroneous results. However, the use of 15N-labelled arginine as substrate and measurement of 15N-labelled agmatine is a direct, accurate and sensitive method for determination of the ADC activity.
Results of the present study indicate that the content of agmatine in freeze-clamped intact livers (baseline level without perfusion) and in control-perfused livers are 10–15-fold lower than what we had previously reported in [10]. This difference may be due to a lower concentration in livers obtained from overnight-fasted rats (the present study) versus fed rats [10]. Consumption of agmatine from dietary sources would be expected to increase hepatic [agmatine] in fed versus overnight-fasted rats. In addition, in previous studies [agmatine] was measured by HPLC [10], rather than the isotope dilution methodology used in the present study. It is possible that the measurement of agmatine levels by HPLC resulted in an overestimation of tissue [agmatine] due to an incomplete separation from other tissue constituents.
An observation of special interest is that insulin and glucagon had no significant effect on the output of [guanidino-15N2]agmatine, whereas dibutyryl-cAMP significantly (P<0.05) stimulated the output of [guanidino-15N2]agmatine during liver perfusion with [guanidino-15N2]arginine (Figure 4). Both glucagon and insulin are established regulators of hepatic amino acid uptake and metabolism. In most cases, the action of glucagon is brought about by cAMP [31,33]. Previous studies by O'Sullivan et al. [31] have shown that both glucagon and dibutyryl-cAMP stimulated the release of 14CO2 during liver perfusion with [U-14C]arginine but not from [U-14C]ornithine. The observation of the present study showing that dibutyryl-cAMP stimulated the flux through the ADC reaction is in line with the stimulation of 14CO2 release during liver perfusion with [U-14C]arginine [31].
cAMP regulates numerous physiological functions and enzyme activities, and mediates long- and short-term response mainly through the cAMP/PKA (protein kinase A) signalling pathway and phosphorylation [33,34]. An example is the down-regulation of NO production and the up-regulation of arginase [35]. It is unlikely that the stimulation of arginase affected flux through the ADC reaction because the addition of Mn2+, which significantly stimulated the production of urea by the arginase reaction (results not shown), had minimal effect on flux through ADC. An inhibition of cytosolic NO production by cAMP might affect mitochondrial ADC but it is most probable that cAMP stimulates flux through the ADC reaction by the cAMP/PKA signalling pathway. This possibility is in line with earlier studies suggesting that PKA activity is present in mitochondria [36], and affects the activity of various enzymes [33,34]. Nonetheless, further studies are required to explore the mechanism(s) of the cAMP action on flux through the ADC reaction.
In mammalian tissue, agmatine may be metabolized by the agmatinase reaction to form urea and putrescine or by the diamine oxidase reaction to form guanidinobutanoic acid [3,9,37]. Preliminary GC–MS screening indicates that 15N-labelled putrescine was formed by the mitochondria (results not shown), but 15N-labelled guanidinobutanoic acid was not detected. However, further qualitative and quantitative analyses, including specific GC–MS methods, are required to elucidate mitochondrial agmatine metabolism.
In summary, the findings of the present study demonstrate that 15N-labelled agmatine was generated from exogenous 15N-labelled arginine both in isolated mitochondria and in the liver perfusion system. The observations are in line with earlier studies demonstrating the activity of ADC in mammalian tissue [2,3,5,6,10]. The results suggest that cAMP may regulate the flux through the ADC reaction.
Acknowledgments
This work was supported by the National Institutes of Health grants DK-53761 and CA-79495 (to I. N.).
References
- 1.Rasch W., Regunathan S., Li G., Reis D. J. Agmatine, the bacterial amine, is widely distributed in mammalian tissues. Life Sci. 1995;56:2319–2330. doi: 10.1016/0024-3205(95)00226-v. [DOI] [PubMed] [Google Scholar]
- 2.Regunathan S., Reis D. J. Characterization of arginine decarboxylase in rat brain and liver: distinction from ornithine decarboxylase. J. Neurochem. 2000;74:2201–2208. doi: 10.1046/j.1471-4159.2000.0742201.x. [DOI] [PubMed] [Google Scholar]
- 3.Lortie M. J., Novotny W. F., Peterson O. W., Vallon V., Malvey K., Mendonca M., Satriano J., Insel P., Thomson S. C., Blantz R. C. Agmatine, a bioactive metabolite of arginine: production, degradation, and functional effects in the kidney of the rat. J. Clin. Invest. 1996;97:413–420. doi: 10.1172/JCI118430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Y., Halaris A. E., Piletz J. E. Determination of agmatine in brain and plasma using high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B. 1997;691:277–286. doi: 10.1016/s0378-4347(96)00458-6. [DOI] [PubMed] [Google Scholar]
- 5.Gen L., Regunathan D., Barrow C. J., Esraghi J., Cooper R., Reis D. J. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994;263:12231–12234. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- 6.Blantz R. C., Satriano J., Gabbai F., Kelly C. Biological effects of arginine metabolites. Acta Physiol. Scand. 2000;168:21–25. doi: 10.1046/j.1365-201x.2000.00646.x. [DOI] [PubMed] [Google Scholar]
- 7.Satriano J., Kelly C. J., Blantz R. C. An emerging role for agmatine. Kidney Inter. 1999;56:1252–1253. doi: 10.1046/j.1523-1755.1999.00697.x. [DOI] [PubMed] [Google Scholar]
- 8.Reis D. J., Regunathan S. Is agmatine a novel neurotransmitter in brain? Trends Pharmacol. Sci. 2000;5:187–193. doi: 10.1016/s0165-6147(00)01460-7. [DOI] [PubMed] [Google Scholar]
- 9.Grillo M. A., Colombatto S. Metabolism and function in animal tissues of agmatine, a biogenic amine formed from arginine. Amino Acids. 2004;26:3–8. doi: 10.1007/s00726-003-0030-z. [DOI] [PubMed] [Google Scholar]
- 10.Nissim I., Horyn O., Daikhin Y., Nissim I., Lazarow A., Yudkoff M. Regulation of urea synthesis by agmatine in the perfused liver: studies with 15N. Am. J. Physiol. Endocrinol Metab. 2002;283:E1123–E1134. doi: 10.1152/ajpendo.00246.2002. [DOI] [PubMed] [Google Scholar]
- 11.Wu W. H., Morris D. R. Biosynthetic arginine decarboxylase from Escherichia coli: purification and properties. J. Biol. Chem. 1973;248:1687–1695. [PubMed] [Google Scholar]
- 12.Wu W. H., Morris D. R. Biosynthetic arginine decarboxylase from Escherichia coli: subunit interactions and the role of magnesium ion. J. Biol. Chem. 1973;248:1696–1699. [PubMed] [Google Scholar]
- 13.Morrissey J., McCracken R., Ishidoya S., Klahr S. Partial cloning and characterization of an arginine decarboxylase in the kidney. Kidney Int. 1995;47:1458–1461. doi: 10.1038/ki.1995.204. [DOI] [PubMed] [Google Scholar]
- 14.Zhu M. Y., Iyo A., Piletz J. E., Regunathan S. Expression of human arginine decarboxylase, the biosynthetic enzyme for agmatine. Biochim. Biophys. Acta. 2004;1670:156–164. doi: 10.1016/j.bbagen.2003.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman C. S., Hu G., Pegg A. E. Putrescine biosynthesis in mammalian tissues. Biochem. J. 2004;379:849–855. doi: 10.1042/BJ20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vatamaniuk M. Z., Horyn O. V., Vatamaniuk O. K., Doliba N. M. Acetylcholine affects rat liver metabolism via type 3 muscarinic receptors in hepatocytes. Life Sci. 2003;7:1871–1882. doi: 10.1016/s0024-3205(02)02506-7. [DOI] [PubMed] [Google Scholar]
- 17.Cohen N. S., Cheung C. W., Kyan F. S., Jones E. E., Raijman L. Mitochondrial carbamyl phosphate and citrulline synthesis at high matrix acetylglutamate. J. Biol. Chem. 1982;257:6898–6907. [PubMed] [Google Scholar]
- 18.Morris S. M., Jr Recent advances in arginine metabolism. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:45–51. doi: 10.1097/00075197-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Wu G., Morris S. M. Arginine metabolism: nitric oxide and beyond. Biochem. J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colleluori D. M., Morris S. M., Ash D. E. Expression, purification, and characterization of human type II arginase. Arch. Biochem. Biophys. 2001;389:135–143. doi: 10.1006/abbi.2001.2324. [DOI] [PubMed] [Google Scholar]
- 21.Szweda L. I., Atkinson D. E. Response of rat liver glutaminase to pH: mediation by phosphate and ammonium ions. J. Biol. Chem. 1989;264:15357–15360. [PubMed] [Google Scholar]
- 22.Chance B., Williams G. R. A simple and rapid assay of oxidative phosphorylation. Nature (London) 1955;175:1120–1121. doi: 10.1038/1751120a0. [DOI] [PubMed] [Google Scholar]
- 23.Nissim I., Horyn O., Luhovyy B., Lazarow A., Daikhin Y., Nissim I., Yudkoff M. Role of the glutamate dehydrogenase reaction in furnishing aspartate nitrogen for urea synthesis: studies in perfused rat liver with 15N. Biochem. J. 2003;376:179–188. doi: 10.1042/BJ20030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stickle D., Bohrer A., Berger R., Morrissey J., Klahr S., Turk J. Quantitation of the putative neurotransmitter agmatine as the hexafluoroacetylacetonate derivative by stable isotope dilution gas chromatography and negative-ion chemical ionization mass spectrometry. Anal. Biochem. 1996;238:129–136. doi: 10.1006/abio.1996.0265. [DOI] [PubMed] [Google Scholar]
- 25.Nissim I., Yudkoff M., Brosnan J. T. Regulation of [15N] urea synthesis from [5-15N] glutamine: role of pH, hormones and pyruvate. J. Biol. Chem. 1996;271:31234–31242. doi: 10.1074/jbc.271.49.31234. [DOI] [PubMed] [Google Scholar]
- 26.Jones B. N., Gilligan J. P. Ortho-phthaldialdehyde precolumn derivatization and reversed-phase high-performance liquid-chromatography of polypeptide hydrolysates and physiological fluid. J. Chromatogr. 1983;266:471–482. doi: 10.1016/s0021-9673(01)90918-5. [DOI] [PubMed] [Google Scholar]
- 27.Medina M. A., Urdiales J. L., Rodriguez-Caso C., Ramirez F. J., Sanchez-Jimenez F. Biogenic amines and polyamines: similar biochemistry for different physiological missions and biomedical applications. Crit. Rev. Biochem. Mol. Biol. 2003;38:23–59. doi: 10.1080/713609209. [DOI] [PubMed] [Google Scholar]
- 28.Dolinska M., Albrecht J. L-arginine uptake in rat cerebral mitochondria. Neurochem. Int. 1998;33:233–236. doi: 10.1016/s0197-0186(98)00020-5. [DOI] [PubMed] [Google Scholar]
- 29.Freedland R. A., Crozier G. L., Hicks B. L., Meijer A. J. Arginine uptake by isolated rat liver mitochondria. Biochim. Biophys. Acta. 1984;802:407–412. doi: 10.1016/0304-4165(84)90357-x. [DOI] [PubMed] [Google Scholar]
- 30.O'Sullivan D., Brosnan J. T., Brosnan M. E. Hepatic zonation of the catabolism of arginine and ornithine in the perfused rat liver. Biochem. J. 1998;330:627–632. doi: 10.1042/bj3300627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Sullivan D., Brosnan J. T., Brosnan M. E. Catabolism of arginine and ornithine in the perfused rat liver: effect of dietary protein and of glucagon. Am. J. Physiol. 2000;278:E516–E521. doi: 10.1152/ajpendo.2000.278.3.E516. [DOI] [PubMed] [Google Scholar]
- 32.Schipper R. G., Verhofstad A. A. Distribution patterns of ornithine decarboxylase in cells and tissues: facts, problems, and postulates. J. Histochem. Cytochem. 2002;50:1143–1160. doi: 10.1177/002215540205000901. [DOI] [PubMed] [Google Scholar]
- 33.Houslay M. D. Compartmentalization of cyclic AMP, phosphodiesterases, signaling crosstalk, desensitization and the phosphorylation of G1-2 and cell specific personalization to the control of the levels of the second messenger cyclic. AMP. Adv. Enzyme Regul. 1995;35:303–338. doi: 10.1016/0065-2571(94)00012-r. [DOI] [PubMed] [Google Scholar]
- 34.Shabb J. B. Physiological substrates of cAMP-dependent protein kinase. Chem. Rev. 2001;101:2381–2411. doi: 10.1021/cr000236l. [DOI] [PubMed] [Google Scholar]
- 35.Chang C.-I., Zoghi B., Liao J. C., Kuo L. The involvement of tyrosine kinases, cyclic AMP/protein kinase A, and p38 mitogen-activated protein kinase in IL-13-mediated arginase I induction in macrophage: its implication in IL-13-inhibited nitric oxide production. J. Immunol. 2000;165:2134–2141. doi: 10.4049/jimmunol.165.4.2134. [DOI] [PubMed] [Google Scholar]
- 36.Technikova-Dobrova Z., Sardanelli A. M., Speranza F., Scacco S., Signorile A., Lorusso V., Papa S. Cyclic adenosine monophosphate-dependent phosphorylation of mammalian mitochondrial proteins: enzyme and substrate characterization and functional role. Biochemistry. 2001;40:13941–13947. doi: 10.1021/bi011066p. [DOI] [PubMed] [Google Scholar]
- 37.Dallmann K., Junker H., Balabanov S., Zimmermann U., Giebel J., Walther R. Human agmatinase is diminished in the clear cell type of renal cell carcinoma. Int. J. Cancer. 2004;108:342–347. doi: 10.1002/ijc.11459. [DOI] [PubMed] [Google Scholar]