FIGURE 5.
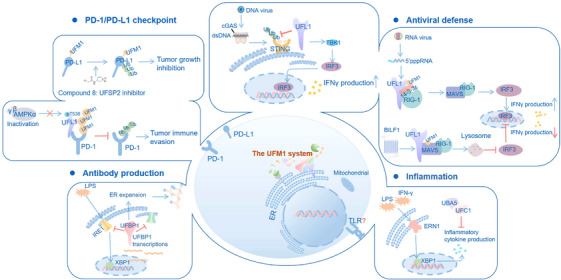
The function of UFMylation in immune response. UFMylation controls adaptive immunity through modulating PD‐1/PD‐L1 checkpoint and B‐cell development and function. UFMylation tags PD‐L1 for proteasome‐mediated degradation. 10 Depletion of UFM1 components stabilises PD‐L1 through inhibiting its degradation. UFL1 can interact with PD‐1 to promote its UFMylation, antagonising PD‐1 ubiquitination and degradation. 11 UFL1 ablation in T cells reduces PD‐1 UFMylation, destabilising PD‐1 and enhancing CD8+ T‐cell‐mediated anti‐tumour response. The differentiation of plasma B cells stimulates a transcriptional increase of UFBP1 through XBP1s pathway. 12 Serving as a feedback inhibitor, UFBP1 constrains the activity of sensors IRE1 and PERK, fostering ER expansion and immunoglobulin production. In addition, UFMylation plays critical roles in innate immunity depending on the stimuli and cellular background. UFL1 interacts with STING to prevent its ubiquitination and proteasomal degradation in response to HSV‐1 infection in peritoneal macrophages. 9 In 293T cells infected with SenV, increased interaction between 14‐3‐3ε and UFL1 leads to UFMylation and subsequent binding to K63‐ubiquitinated RIG‐Ι, enhancing downstream IFN signalling. 7 During EBV replication in Burkitt lymphoma cell lines, the EBV‐encoded BILF1 mediates MAVS UFMylation, targeting it for lysosomal degradation and potentially disrupting RIG‐I‐MAVS‐driven NLRP3 inflammasome activation and IRF3 responses. 8 Moreover, the enzymatic activities of UFSP2, UBA5 and UFC1 are required in the negative regulation of macrophage activation mediated by IFN‐γ and LPS. 13 Transcriptional profiling in cells with deficient UFMylation has revealed an ERN1‐mediated ER stress response, which accounts for the increased sensitivity to pro‐inflammatory stimuli.
