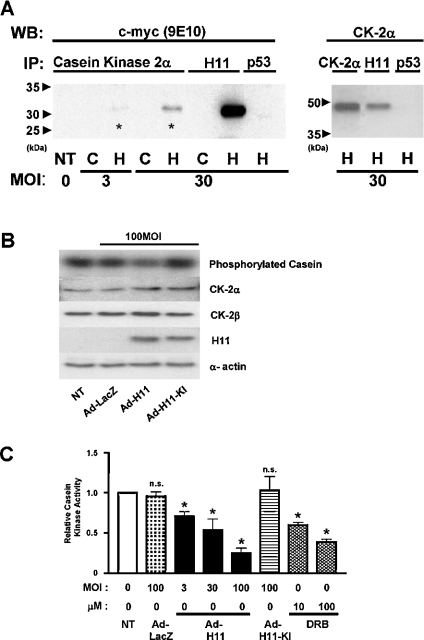
Figure 6. H11 physically associates with CK2α.
(A) Cardiac myocytes were transduced with Ad-H11-myc (‘H’) or control Ad-LacZ (‘C’). Cell lysates were subjected to immunoprecipitation (IP) with an antibody against CK2α, H11 or p53. The immunoprecipitates were then subjected to immunoblot analysis with anti-c-myc (9E10) antibody (left panel) or anti-CK2α antibody (right). Asterisk indicates the H11myc co-immunoprecipitated with CK2α. NT, no transduction; WB, Western blot. (B, C) Myocytes were transduced with Ad-LacZ, Ad-H11 or Ad-H11-KI at MOI=100 for 48 h; 24 h after transduction, myocytes were treated with DRB at the indicated concentrations for 24 h. The kinase activity of CK2 was evaluated by immunocomplex kinase assays using casein as a substrate (B, top panel). A part of the cell lysate was subjected to immunoblot analyses with anti-CK2α, CK2β, H11 and α-actin antibody, showing that comparable levels of CK2 (α and β) are expressed and that H11 or H11-KI is overexpressed. (C) Phosphorylation of casein was quantified by densitometric analysis. The value from myocytes without adenovirus transduction or DRB was designated as 1.0. Each bar represents the mean for three experiments performed in duplicate. NT, no transduction. *P<0.001 versus NT. n.s., not significant.