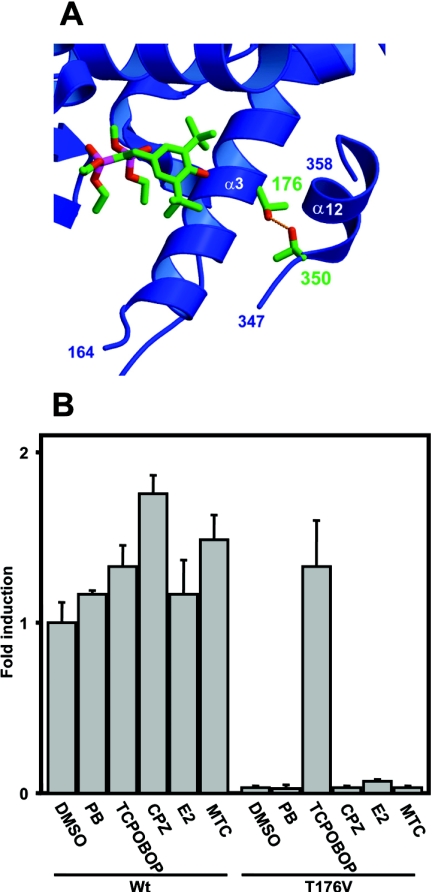
Figure 1. Thr176 of mCAR and its valine substitution.
(A) Interaction of Thr176 with Thr350 in the model structure of mCAR. The ligand molecule in the PXR structure [14] is shown by superimposing it with the CAR structure. Numbers indicate the two conserved threonine residues. (B) The wild-type mCAR (Wt) and the Thr176→Val (T176V) mutant were co-transfected into HepG2 cells with (NR1)5-tk-luciferase plasmid (0.1 μg) and pRL-SV40 (0.01 μg), which is an R. reniformis luciferase expression plasmid for normalization of transfection efficiency. After 16 h, cells were subsequently treated with phenobarbital (PB; 1 mM), TCPOBOP (250 nM), chlorpromazine (CPZ, 10 μM), oestradiol (E2, 10 μM) or methoxychlor (MTC, 10 μM) for an additional 24 h before preparation of lysates for luciferase assay. The fold activation was calculated relative to the activity with DMSO for the wild-type receptor as 1. The results are expressed as the means±S.D. from three independent experiments.