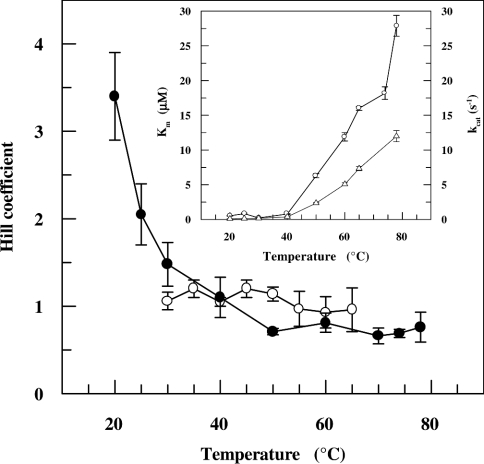
Figure 4. Dependence of kinetic co-operativity of SsADH and mSsADH on temperature.
Hill coefficient values for SsADH (●) were calculated from the steady-state kinetic parameters determined with a saturating concentration of benzaldehyde and variable NADH concentrations in 50 mM glycine/NaOH, pH 9.8, using fluorescence and spectrophotometric methods at temperatures below and above 40 °C respectively. The Hill coefficient values for mSsADH (○) were calculated from the steady-state kinetic parameters determined with a saturating concentration of cyclohexanol and variable NAD+ concentrations in 50 mM glycine/NaOH, pH 9.8, as described under the Experimental section. Inset, the dependence of Km (○) and kcat (△) of SsADH for NADH on temperature. The results are expressed as the means±S.D. and are smaller than the symbol when absent in the Figure.