ABSTRACT
Objectives
Myocardial infarction without significant stenosis or occlusion of the coronary arteries carries a high risk of recurrent major adverse cardiovascular events and poor prognosis. This study aimed to investigate the association between body mass index and outcomes in patients with a suspected myocardial infarction with nonobstructive coronary artery disease (MINOCA).
Methods
Patients were recruited at Bergmannsheil University Hospital from January 2010 to April 2021. The primary outcomes were in‐hospital and long‐term mortality. Secondary outcomes consisted of adverse events during hospitalization and during follow‐up.
Results
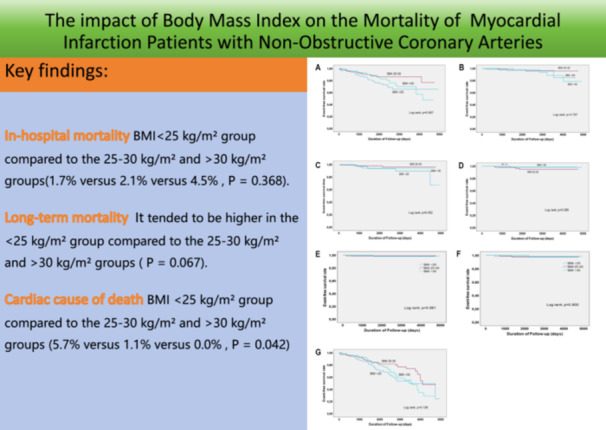
A total of 373 patients were included in the study, with a mean follow‐up time of 6.2 years. The patients were divided into different BMI groups: < 25 kg/m² (n = 121), 25−30 kg/m² (n = 140), and > 30 kg/m² (n = 112). In‐hospital mortality was 1.7% versus 2.1% versus 4.5% (p = 0.368). However, long‐term mortality tended to be higher in the < 25 kg/m² group compared to the 25−30 and > 30 kg/m² groups (log‐rank p = 0.067). Subgroup analysis using Kaplan−Meier analysis showed a higher rate of cardiac cause of death in the < 25 kg/m² group compared to the 25−30 and > 30 kg/m² groups: 5.7% versus 1.1% versus 0.0% (log‐rank p = 0.042). No significant differences were observed in other adverse events between the different BMI groups during hospitalization and long‐term follow‐up.
Conclusions
Patients with a BMI < 25 kg/m² who experience a suspected myocardial infarction without significant coronary artery disease may have higher all‐cause mortality and cardiovascular cause of death. However, further data are needed to confirm these findings.
Keywords: adverse events, mortality, myocardial infarction, obesity, overweight
In patients with myocardial infarction without significant coronary artery disease, a body mass index < 25 kg/m2 is associated with higher all‐cause mortality and cardiovascular cause of death.
1. Introduction
Myocardial infarction with nonobstructive coronary arteries (MINOCA) is a subtype of myocardial infarction (MI) and accounts for approximately 6% (ranging from 5% to 15%) of MI patients who undergo coronary angiography [1, 2, 3, 4]. It was first mentioned nearly 80 years ago [5] and is defined as an acute myocardial infarction (AMI) without a significant coronary artery obstruction on angiography (≥ 50% diameter stenosis in a major epicardial vessel) or specific imaging findings according to the Fourth Universal Definition of MI [6]. Multiple studies have confirmed that MINOCA has a lower mortality rate than acute myocardial infarction with obstructive coronary artery disease (MI‐CAD), but it still carries a high risk of recurrent major adverse cardiovascular events (MACE) and poor prognosis compared to healthy individuals [4, 7, 8]. The prevalence of MINOCA is higher in females and younger individuals [3, 9], and MACE has been associated with cardiovascular risk factors such as smoking, hypertension, and diabetes mellitus [3].
Obesity is a risk factor for metabolic diseases and contributes to atherosclerosis and CAD [10, 11, 12]. The prevalence of overweight and obesity has rapidly increased in recent years [13]. Obesity has been linked to a higher risk of MI and ischemic heart disease, with or without metabolic syndrome [14]. However, the concept of the obesity paradox has emerged in recent years, as several relevant studies have shown that obesity is associated with better cardiovascular disease prognosis compared to individuals of normal weight [15, 16, 17]. Additionally, a recent study found that normal‐weight individuals with diabetes had comparable adjusted cardiovascular mortality to obese individuals without diabetes [18]. Another study revealed that patients with a body mass index (BMI) less than 18.5 kg/m2 had higher mortality rates than both normal weight and obese patients with AMI [19].
While myocardial infarction is a fatal disease and patients suffer from acute chest pain and persistent ECG changes associated with elevated troponins and in a coronary angiography a culprit lesion is confirmed. MINOCA is a syndrome with multiple etiologies from cardiac and noncardiac causes and is commonly seen in hospitalized patients diagnosed with MI. Therefore, an accurate and systematic examination is needed to help identify the cause of MINOCA in different patients, stratify the risk and implement the most appropriate treatment. Patients with MINOCA, especially those with normal coronary angiography, are often misdiagnosed as “non‐cardiac patients,” missing the optimal treatment opportunity and affecting patient prognosis. Most studies concentrated on the association between obesity and myocardial infarction and confirmed that obesity should be an important risk factor. MINOCA is an important subtype of which has a different mechanism. With the increasing diagnosis of MINOCA, there is no relevant data analysis on whether obesity is related to it. And no relevant studies have focused on the association between BMI and prognosis in patients with MINOCA. Therefore, the present study aims to evaluate the association between BMI and the clinical presentation and outcomes of patients with suspected MINOCA in a large cohort.
2. Methods and Materials
2.1. Study Population
From January 2010 to April 2021, a total of 24 775 patients who underwent coronary angiography at Bergmannsheil University Hospital were enrolled. All participants aged ≥ 18 years old were eligible for the study, and those with incomplete information (e.g., missing ventilation protocols) were excluded. The study protocol received approval from the Ethics Committee of the Medical Faculty of Ruhr University Bochum (22‐7684). The informed consent was obtained from each patient and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
2.2. Data Collection
Three hundred and seventy‐three had elevated troponin values without significant coronary artery stenosis confirmed by coronary angiography. Cardiac troponin levels needed to be elevated, with at least one value surpassing the 99th percentile. All demographic information and cardiovascular risk factors were collected from medical records. Coronary angiography results, and treatment approach in the hospital were documented during the hospitalization period. We also collected medication information used at the time of admission and discharge.
2.3. Definitions and Outcomes
The inclusion based on assessments of laboratory reports, ECGs, and angiograms. The BMI was calculated as weight in kilograms divided by the square of height in meters. Overweight was defined as a BMI between 25.0 and 30.0 kg/m2, while obesity was defined as a BMI > 30.0 kg/m2 according to guidelines [20].
The major adverse events in the hospital were defined as a combination of cardiopulmonary resuscitation, left ventricular thrombus, thromboembolic events, pulmonary edema, cardiogenic shock, invasive and noninvasive ventilation, stroke, life‐threatening cardiac arrhythmias, supraventricular arrhythmias, and overall mortality. We also divided in‐hospital death into cardiac‐caused and noncardiac‐caused as far as the cause of death was found. During the follow‐up period, long‐term outcomes (including stroke, thromboembolic events, recurrence of MINOCA, cardiac arrest, percutaneous coronary intervention [PCI], and all‐cause mortality) were documented. And we also recorded cardiac‐caused death and noncardiac‐caused death.
The primary outcomes were defined as in‐hospital and long‐term mortality. The second outcomes were other adverse outcomes.
2.4. Statistics Analysis
Mean ± standard deviation was used in the continuous variables with normal distribution, while median (interquartile range) was used in the continuous variables with non‐normal distribution. Differences in demographic and cardiovascular risk factors between different BMI groups were tested using the Students t‐test, chi‐square test, or analysis of variance. The cumulative incidence of adverse events among the groups was shown by Kaplan−Meier analysis and compared using the log‐rank test. Statistically significant was declared if two‐sided p < 0.05, and data were analyzed using SPSS Statistics 29.0 software.
3. Results
3.1. Basic Characteristics
A total of 373 patients were enrolled in the study, and the mean follow‐up time was 6.2 years. The flowchart is shown in Figure 1. The average age of the patients was 63 ± 15.6 years old, and the proportion of females was 50.4%. Among the patients, 121 (32.4%) were in the BMI < 25 kg/m² group, 140 (37.5%) were overweight (BMI 25−30 kg/m²), and 112 (30.0%) were obese (BMI > 30 kg/m²). There were no significant differences in age between the groups, but the BMI < 25 kg/m² group had a higher proportion of females (p < 0.05, Table 1).
Figure 1.
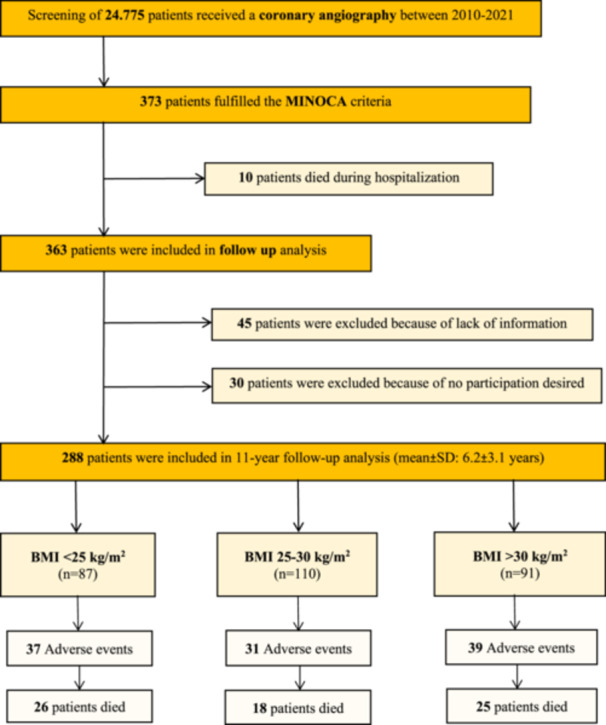
Flowchart presenting the screened data and included patients for the present study.
Table 1.
Baseline characteristics of 373 patients initially presenting MINOCA according to BMI.
| Variables | All patients n = 373 | < 25 n = 121 | 25−30 n = 140 | > 30 n = 112 | p value |
|---|---|---|---|---|---|
| Age—years, mean ± SD | 63 ± 15.6 | 62.5 ± 17 | 62.5 ± 17 | 63 ± 14 | 0.839 |
| Male—n (%) | 185 (49.6) | 48 (39.7) | 78 (55.7) | 59 (52.7) | 0.026 |
| Duration of hospitalization—days, mean ± SD | 10 ± 8.5 | 9 ± 6 | 9 ± 6 | 10.1 ± 7 | 0.009 |
| Symptoms—n (%) | |||||
| Angina pectoris | 226 (61.6) | 76 (64.4) | 82 (59.4) | 68 (61.3) | 0.715 |
| Dyspnea | 164 (44.4) | 55 (46.6) | 50 (35.7) | 59 (53.2) | 0.019 |
| Palpations | 45 (12.3) | 13 (11) | 21 (15.2) | 11 (9.9) | 0.396 |
| Clinic parameter, mean ± SD | |||||
| Systolic BP, mmHg | 146.6 ± 64.4 | 153.5 ± 98 | 153.5 ± 98 | 147.3 ± 29 | 0.235 |
| Diastolic BP, mmHg | 84.8 ± 18.1 | 82.9 ± 18 | 82.9 ± 18 | 87.2 ± 19 | 0.268 |
| Heart rate, bpm | 89.8 ± 28.6 | 87 ± 26 | 86.8 ± 26 | 95.6 ± 31 | 0.041 |
| ECG—n (%) | |||||
| ST elevation | 55 (14.8) | 23 (19) | 14 (10) | 18 (16.1) | 0.111 |
| Inversed T‐waves | 183 (49.2) | 67 (55.8) | 70 (50) | 46 (41.1) | 0.078 |
| Medical history—n (%) | |||||
| Current smoking | 85 (23.1) | 23 (19.2) | 38 (27.3) | 24 (22) | 0.285 |
| Arterial hypertension | 253 (68.2) | 74 (61.2) | 96 (68.6) | 83 (75.5) | 0.066 |
| Dyslipidemia | 99 (26.6) | 23 (19) | 41 (29.3) | 35 (31.5) | 0.065 |
| Diabetes mellitus | 65 (17.5) | 10 (8.3) | 18 (12.9) | 37 (33.3) | < 0.001 |
| COPD | 47 (12.6) | 25 (20.7) | 10 (7.1) | 12 (10.8) | 0.004 |
| Bronchial asthma | 33 (8.9) | 5 (4.1) | 18 (12.9) | 10 (9) | 0.047 |
| Malignancy | 47 (12.7) | 19 (15.8) | 16 (11.4) | 12 (10.9) | 0.455 |
| Kidney disease | 53 (14.3) | 15 (12.4) | 17 (12.1) | 21 (18.9) | 0.244 |
| Neurological disease | 90 (24.3) | 40 (33.1) | 24 (17.1) | 26 (23.6) | 0.011 |
| Autoimmune disease | 17 (4.6) | 5 (4.1) | 7 (5) | 5 (4.6) | 0.946 |
| Psychiatric disease | 39 (10.5) | 8 (6.6) | 19 (13.6) | 12 (10.8) | 0.187 |
| Pacemaker | 14 (3.8) | 4 (3.3) | 7 (5) | 3 (2.7) | 0.613 |
| Supraventricular arrhythmiasa | 57 (15.4) | 23 (19.2) | 21 (15) | 13 (11.8) | 0.316 |
| Atrial fibrillation | 57 (15.4) | 23 (19.2) | 21 (15) | 13 (11.8) | 0.316 |
| Atrial flutter | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 |
| Laboratory values, mean ± SD | |||||
| Troponin (µg/L) | 2.1 ± 8.4 | 1.6 ± 5.5 | 1.3 ± 7 | 2.6 ± 9.8 | 0.435 |
| Creatin phosphatkinase (U/L) | 288.6 ± 548.9 | 282.5 ± 501 | 260.1 ± 451 | 330.6 ± 692 | 0.594 |
| BNP (pg/mL) | 433.5 ± 1004.5 | 598 ± 1271 | 378.3 ± 1056 | 323.5 ± 479 | 0.187 |
| Creatinine (mg/dL) | 3.3 ± 30.9 | 5.7 ± 51.7 | 1.1 ± 0.7 | 3.5 ± 17 | 0.483 |
| Echocardiography data, n (%) | |||||
| LVEF % (on admission), mean ± SD | 36.9 ± 25.7 | 37.9 ± 26.4 | 37.6 ± 25.4 | 34.93 ± 25 | 0.621 |
| LVEF ≥ 50% | 170 (66.9) | 54 (64.3) | 69 (72.6) | 47 (62.7) | 0.323 |
| LVEF 49%−40% | 31 (12.2) | 14 (16.7) | 8 (8.4) | 9 (12) | 0.245 |
| LVEF < 40% | 52 (20.5) | 15 (17.9) | 18 (19) | 19 (25.3) | 0.458 |
| Left ventricular hypertrophy | 103 (29.1) | 33 (28.7) | 30 (22.7) | 40 (37.4) | 0.046 |
| Tricuspid valve regurgitation | 89 (24.2) | 28 (23.5) | 39 (28.1) | 22 (20) | 0.332 |
| ‐ Mild | 63 (17.1) | 19 (16) | 28 (20.2) | 16 (14.6) | 0.470 |
| ‐ Moderate | 23 (6.3) | 8 (6.7) | 10 (7.2) | 5 (4.6) | 0.671 |
| ‐ Severe | 3 (0.8) | 1 (0.8) | 1 (0.7) | 1 (0.9) | 0.986 |
| Mitral valve regurgitation | 106 (28.8) | 33 (27.7) | 43 (30.9) | 30 (27.3) | 0.780 |
| ‐ Mild | 77 (20.9) | 22 (18.3) | 32 (23) | 23 (20.9) | 0.653 |
| ‐ Moderate | 19 (5.2) | 9 (7.5) | 5 (3.6) | 5 (4.6) | 0.347 |
| ‐ Severe | 10 (2.7) | 2 (1.7) | 6 (4.3) | 2 (1.8) | 0.337 |
| Aortic valve regurgitation | 39 (10.6) | 14 (11.8) | 16 (11.5) | 9 (8.2) | 0.617 |
| ‐ Mild | 31 (8.4) | 11 (9.2) | 13 (9.4) | 7 (6.4) | 0.651 |
| ‐ Moderate | 4 (1.1) | 1 (0.8) | 1 (0.7) | 2 (1.8) | 0.676 |
| ‐ Severe | 4 (1.1) | 2 (1.7) | 2 (1.4) | 0 (0) | 0.417 |
| Drugs on admission, n (%) | |||||
| β‐Blocker | 131 (35.2) | 47 (38.8) | 40 (28.8) | 44 (39.3) | 0.134 |
| ACE inhibitor | 121 (32.6) | 44 (36.4) | 35 (25.4) | 42 (37.5) | 0.071 |
| Sartane | 57 (15.3) | 12 (9.9) | 27 (19.4) | 18 (16.1) | 0.102 |
| Ca‐Blocker | 74 (19.9) | 22 (18.2) | 24 (17.3) | 28 (25) | 0.266 |
| Diuretics | 101 (27.2) | 29 (24) | 32 (23) | 40 (35.7) | 0.051 |
| Anticoagulantsb | 58 (15.6) | 27 (22.3) | 18 (13) | 13 (11.6) | 0.044 |
| Aspirin | 79 (21.2) | 31 (25.6) | 24 (17.3) | 24 (21.4) | 0.260 |
| Clopidogrel | 18 (4.8) | 9 (7.4) | 3 (2.2) | 6 (5.4) | 0.135 |
| Prasugrel | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 |
| Antiarrhythmicsc | 10 (2.7) | 2 (1.7) | 5 (3.6) | 3 (2.7) | 0.629 |
Abbreviations: ACE, angiotensin‐converting‐enzyme; AV, atrioventricular; BMI, body mass index; BNP, brain natriuretic peptide; BP, blood pressure; COPD, chronic obstructive pulmonary disease; ECG, electrocardiogram; HFmrEF, heart failure with mid range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LV EF, ejection fraction; MINOCA myocardial infarction with nonobstructive coronary artery disease; SD, standard deviation.
Only one malignant cardiac/supraventricular arrhythmia is counted per patient (even if one patient has several arrhythmias at the same time).
Cumarine, heparin, selective factor 10‐blocker, direct thrombin inhibitors.
Ivabradine, flecainid, sotalol, dronedaron, digitalis.
p values for the comparison between groups of ages.
Among the patients included in the registry, the results indicated that the obesity (BMI > 30 kg/m²) group had the longest hospitalization days and the highest proportion of dyspnea (p < 0.05). Additionally, the heart rate was higher in the BMI > 30 kg/m² group (95.6 ± 31 bpm) compared to the other groups (BMI 25−30 kg/m² 86.8 ± 26 bpm and < 25 kg/m² 87 ± 26 bpm; p = 0.04).
Regarding medical histories, the obesity group had the highest proportion of diabetes mellitus, while the BMI < 25 kg/m² group had the highest proportion of neurological diseases and chronic obstructive pulmonary disease (COPD) (p < 0.05). There were no significant differences in the other medical histories. All patients enrolled had an echocardiography in the hospital, and the results suggested that the obesity group had the most severe left ventricular hypertrophy (p < 0.05). The left ventricular ejection fraction was similar in all groups. Laboratory values such as troponin, creatinine kinase, and BNP were similar in all groups. Drugs at discharge were similar in all groups (Supporting Information S1: Table 1).
3.2. Primary Outcomes
3.2.1. Association Between BMI and In‐Hospital Mortality and Long‐Term Mortality
In‐hospital death occurred in 10 (2.7%) patients, 6 (1.6%) due to cardiac causes and 4 (1.7%) due to noncardiac causes (Table 2). The in‐hospital mortality rates were as follows: 2 (1.7%) in the BMI < 25 kg/m² group, 3 (2.1%) in the BMI 25−30 kg/m² group, and 5 (4.5%) in the BMI > 30 kg/m² group; p = 0.368 (Supporting Information S1: Figure 1). During the follow‐up period, 69 (24.1%) patients died. The rates of all‐cause mortality were 29.9% in the BMI < 25 kg/m² group, 16.7% in the BMI 25−30 kg/m² group, and 27.5% in the BMI > 30 kg/m² group; p= 0.067. Subgroup analysis using Kaplan−Meier analysis revealed a weak correlation between the obesity group and an increased risk of cardiac death (log‐rank p= 0.042; Table 3). No significant differences were observed in noncardiac mortality rates.
Table 2.
In‐hospital complications according to BMI.
| All patients n = 373 | < 25 n = 121 | 25−30 n = 140 | > 30 n = 112 | p value | |
|---|---|---|---|---|---|
| Adverse event | 132 (35.5) | 39 (32.2) | 50 (35.7) | 43 (38.4) | 0.616 |
| CPR | 7 (1.9) | 1 (0.8) | 4 (2.9) | 2 (1.8) | 0.484 |
| Left ventricular thrombus | 3 (0.8) | 0 (0) | 2 (1.4) | 1 (0.9) | 0.435 |
| Thromboembolic event | 3 (0.8) | 1 (0.8) | 2 (1.4) | 0 (0) | 0.453 |
| Pulmonary edema | 9 (2.7) | 3 (2.5) | 3 (2.1) | 3 (2.7) | 0.961 |
| Cardiogenic shock | 9 (2.7) | 3 (2.5) | 3 (2.1) | 3 (2.7) | 0.961 |
| Invasive ventilation | 29 (7.8) | 7 (5.8) | 11 (7.9) | 11 (9.8) | 0.518 |
| Noninvasive ventilation | 11 (3) | 4 (3.3) | 3 (2.1) | 4 (3.6) | 0.771 |
| Stroke | 1 (0.3) | 0 (0) | 1 (0.7) | 0 (0) | 0.436 |
| Malignant cardiac arrhythmias (on admission/in hospital) | 39 (10.5) | 9 (7.4) | 13 (9.3) | 17 (15.2) | 0.133 |
| Bradycardiac arrhythmias | 13 (3.5) | 4 (3.3) | 3 (2.1) | 6 (5.4) | 0.383 |
| ‐ AV block 2 Mobitz | 3 (0.8) | 0 (0) | 2 (1.4) | 1 (0.9) | 0.435 |
| ‐ AV block 3 | 1 (0.3) | 0 (0) | 1 (0.7) | 0 (0) | 0.436 |
| ‐ Asystole | 10 (2.7) | 4 (3.3) | 1 (0.7) | 5 (4.5) | 0.164 |
| Ventricular arrhythmias | 14 (3.8) | 3 (2.5) | 5 (3.6) | 6 (5.4) | 0.510 |
| ‐ Sustained | 8 (2.2) | 3 (2.5) | 1 (0.7) | 4 (3.6) | 0.290 |
| ‐ Non‐sustained | 6 (1.6) | 0 (0) | 3 (2.1) | 3 (2.7) | 0.291 |
| ‐ Ventricular fibrillation | 16 (4.3) | 3 (2.5) | 7 (5) | 6 (5.4) | 0.487 |
| Torsades de pointes | 2 (0.5) | 1 (0.8) | 1 (0.7) | 0 (0) | 0.650 |
| Supraventricular arrhythmias | 85 (22.8) | 30 (24.8) | 32 (22.9) | 23 (20.5) | 0.742 |
| Atrial fibrillation | 77 (20.6) | 28 (23.1) | 27 (19.3) | 22 (19.6) | 0.711 |
| ‐ First appearance | 43 (11.5) | 13 (10.7) | 16 (11.4) | 14 (12.5) | 0.915 |
| ‐ Recurrence | 34 (9.1) | 15 (12.4) | 11 (7.9) | 8 (7.1) | 0.308 |
| Atrial flutter | 9 (2.4) | 2 (1.7) | 5 (3.6) | 2 (1.8) | 0.529 |
| ‐ First appearance | 9 (2.4) | 2 (1.7) | 5 (3.6) | 2 (1.8) | 0.529 |
| ‐ Recurrence | 0 (0) | 0 (0) | 0 (0) | 0 (0) | — |
| In‐hospital death | 10 (2.7) | 2 (1.7) | 3 (2.1) | 5 (4.5) | 0.368 |
| Cardiac caused death | 6 (1.6) | 1 (0.8) | 3 (2.1) | 2 (1.8) | 0.692 |
| Noncardiac caused death | 4 (1.1) | 1 (0.8) | 0 (0) | 3 (2.7) | 0.116 |
Abbreviations: Adverse event, major adverse cardiac and cerebrovascular events; CPR, cardiopulmonary resuscitation; ECMO, extracorporal.
Table 3.
Extra‐hospital complications (during follow‐up) according to BMI.
| All patients n = 288 | < 25 n = 87 | 25−30 n = 110 | > 30 n = 91 | p value | |
|---|---|---|---|---|---|
| Adverse event | 100 (34.8) | 34 (39.1) | 30 (27.5) | 36 (39.6) | 0.126 |
| Stroke | 10 (4.2) | 3 (4.4) | 3 (3.2) | 4 (5.3) | 0.797 |
| Thromboembolic event | 6 (2.5) | 1 (1.4) | 4 (4.3) | 1 (1.4) | 0.385 |
| Recurrence of troponin‐positive with nonobstructive CAD | 3 (1.3) | 1 (1.4) | 1 (1.1) | 1 (1.4) | 0.981 |
| Percutaneous coronary intervention | 17 (7.2) | 7 (10) | 4 (4.3) | 6 (8.2) | 0.352 |
| Death | 69 (24.1) | 26 (29.9) | 18 (16.7) | 25 (27.5) | 0.067 |
| ‐ Cardiac caused death | 5 (2.1) | 4 (5.7) | 1 (1.1) | 0 (0) | 0.042 |
| ‐ Noncardiac caused death | 12 (5.1) | 5 (7.1) | 1 (1.1) | 6 (8.3) | 0.075 |
Abbreviations: Adverse event, major adverse cardiac and cerebrovascular events; CAD, coronary artery disease; CPR, cardiopulmonary resuscitation; NSTEMI, non‐ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction.
3.3. Second Outcomes
3.3.1. Association Between BMI and Other Adverse Events In‐Hospital and Long‐Term Follow‐Up
As shown in Supporting Information S1: Figure 1 and Table 2, there were no significant differences observed between the different BMI groups and the occurrence of adverse in‐hospital events. These events included a combination of cardiopulmonary resuscitation, left ventricular thrombus, thromboembolic events, pulmonary edema, cardiogenic shock, invasive and noninvasive ventilation, stroke, life‐threatening cardiac arrhythmias, and supraventricular arrhythmias.
During the follow‐up period, the Kaplan−Meier curves indicated that there was no significant correlation found between the occurrence of adverse events (such as stroke, thromboembolic events, recurrence of MINOCA, cardiac arrest, and PCI) among all the groups (Figure 2).
Figure 2.
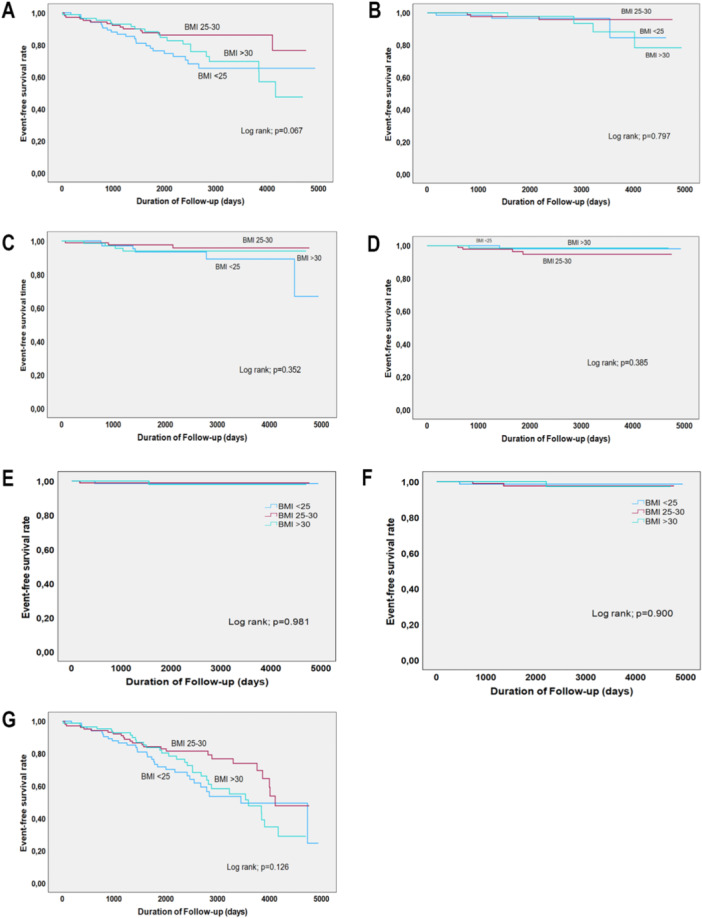
The Kaplan−Meier (KM) curves between adverse events according to different BMI ranges. (A) Shows the KM curve of different BMI ranges and mortality; (B) shows the KM curve of BMI ranges and stroke; (C) shows the KM curve of BMI ranges and percutaneous coronary intervention; (D) shows the KM curve of BMI ranges and thromboembolic events; (E) shows the KM curve of BMI ranges and recurrence of MINOCA; (F) shows the KM curve of BMI ranges and cardiac arrest; and (G) shows the KM curve of BMI ranges and all adverse events.
We also did an extra analysis based on anticoagulant therapy, and the results indicated that during the long‐term follow‐up, the occurrence of adverse events and death were not significantly different between the novel oral anticoagulants (NOACs) treatment group and non‐NOACs treatment group (Supporting Information S1: Table 2).
4. Discussion
In this study, we examined the relationship between BMI and clinical outcomes in patients suspected of MINOCA. Our results indicate that there were no significant differences in adverse events between BMI groups both during hospitalization and in the long‐term. However, when we conducted a subgroup analysis, we found that patients in the obesity group had the lowest prevalence of all‐cause death and cardiac‐caused death.
MINOCA is a cardiovascular disease that poses a high risk for cardiac morbidity and mortality. The prevalence of MINOCA is higher in females and younger people [3, 9]. Consistent with other studies, we found that MINOCA patients tend to be younger. In our study, the enrolled patients were overweight (37.5%) or obese (30%), which was higher than in previous studies [7, 21]. However, the VIRGO study reported an even higher proportion of obesity, up to 42.1% [3], which exceeded our findings. Another recent study involving 233 MINOCA patients showed an average duration of hospitalization [22] of 5 days, whereas our study indicated a duration of 10 days, with the obesity group having the longest hospitalization. Obesity is often accompanied by metabolic diseases [23], and our study showed that patients in the obesity group had a higher proportion of diabetes, higher heart rates, and more symptoms such as dyspnea. These factors may contribute to the longer duration of hospitalization observed in the obesity group compared to other groups.
The in‐hospital mortality rate in our study was 2.7%, with the highest rate observed in the obesity group, although it was lower than in a previous study [24]. However, no significant differences were found between different BMI groups. Previous studies have reported 1‐year mortality rates ranging from 1.1% to 12.5% for MINOCA patients, and a study involving 9092 patients with MINOCA reported a mortality rate of 14% during a mean follow‐up of 4.5 years. In our study, during a mean follow‐up of 6.2 years, 24.1% of the MINOCA patients died. No significant differences were observed between different BMI groups, but the all‐cause mortality and cardiac‐caused death were higher in patients with a BMI < 25 kg/m² compared to those in the overweight and obesity groups. Obesity is often accompanied by metabolic diseases and may increase the incidence of cardiovascular diseases. A pooled analysis of 97 prospective cohorts with 1.8 million participants indicated that each 5 kg/m² increase in BMI was associated with an increased risk ratio of 1.27 for coronary heart disease and 1.18 for stroke [25]. Other studies have reported the obesity paradox, which suggests that overweight and obesity may be associated with a more favorable prognosis for cardiovascular and cerebrovascular diseases [15, 26]. The mechanisms underlying the higher event rate in the BMI 25−30 kg/m² range in MINOCA patients remain unclear, but there are several potential reasons. Non‐purposeful weight loss, greater metabolic reserves, less cachexia, and protective cytokines may all contribute to a better prognosis for cardiovascular events [27]. Some other confounding factors such as dietary habits, physical activity levels, and socioeconomic status were not available in our study, these important factors may also have an effect on the association on BMI and MINOCA.
The adverse outcomes of MINOCA varied depending on the duration of follow‐up in different studies. Previous studies have shown a prevalence of MACE ranging from 4.6% to 23.9% [2, 4, 28], which is lower than what we found in our study. This difference may be due to variations in follow‐up time and inclusion criteria. One study found that 6.3% of MINOCA patients were hospitalized due to recurrent MI over an average follow‐up period of 17 months. Of these patients, 53% had no obstructive CAD and 47% had obstructive CAD [29]. In our study, we observed that during a mean follow‐up of 6.2 years, 1.3% of people were hospitalized due to a recurrence of MI, and 7.2% of patients underwent percutaneous coronary artery intervention. No significant differences were found in terms of in‐hospital or long‐term adverse events among different BMI groups. However, a recent study including 281 patients reported that MINOCA patients with a BMI greater than 25 kg/m2 had a higher prevalence of MACE over a mean follow‐up of 28 months [21], which contradicts our findings. Another study reported that BMI was not associated with MACE [9], although it is worth noting that only one patient in this cohort had a BMI greater than 30 kg/m2.
Our study found that adverse events are comparable over follow‐up in patients treated with NOAC at discharge compared to no NOAC treatment. Due to the limited number of patients, we could not further analyze by BMI grouping, and the number of endpoint events was too small, further studies may be needed to determine the role of anticoagulation therapy in patients with MINOCA.
5. Study Limitations
This monocenter study is important for understanding the relationship between BMI and MINOCA. However, there are several limitations. First, the cross‐sectional design cannot determine causality. Second, the cause of death in many deceased cases during the follow‐up period remained unknown. Third, the definition of MINOCA was solely based on BMI and did not include information on body fat composition, epicardial fat, or muscle mass. Fourth, the small number of endpoints may have influenced the results. Lastly, the monocentric cross‐sectional design with a low number of patients limits the ability to establish causality between BMI and long‐term outcomes in MINOCA patients. Longitudinal studies would provide stronger evidence for causal relationships. Therefore, the conclusions obtained need to be verified by more multicenter studies.
6. Conclusions
In summary, our study revealed an association between BMI and MINOCA. There were no statistically significant differences between overweight, obesity, hospitalization, and the long‐term outcomes of MINOCA. However, in terms of long‐term death and cardiac death, it seems that individuals with a BMI of 25−30 kg/m² had lower mortality and cardiac mortality rates. This finding requires further research to confirm.
Author Contributions
Ibrahim El‐Battrawy and Nazha Hamdani put forwards the design and conception of this study. Mustafa Kacmaz and Clara Schlettert completed data collection. Clara Schlettert participated in the data analysis, and Chaohui Dong completed the manuscript writing. Mohammad Abumayyaleh, Ibrahim Akin, Rayyan Hemetsberger, Andreas Mügge, and Assem Aweimer gave some important suggestions on article revision. All authors have read and agreed to the published vision of the manuscript.
Ethics Statement
The study protocol received approval from the Ethics Committee of the Medical Faculty of Ruhr University Bochum.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Supporting information.
Acknowledgments
This research was funded by EU's Horizon 2020 research and innovation program under grant agreement No. 739593 to N.H.; DFG (Deutsche Forschungsgemeinschaft) HA 7512/2‐4 and HA 7512/2‐1 to N.H.; and a grant from the InnovationForum program of the Medical Faculty, RUB to I.E.‐B. and N.H No. IF‐023‐22 and and No. IF‐034‐22. Open Access funding enabled and organized by Projekt DEAL.
Data Availability Statement
The data can be obtained by contacting the corresponding author. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
- 1. Pasupathy S., Air T., Dreyer R. P., Tavella R., and Beltrame J. F., “Systematic Review of Patients Presenting With Suspected Myocardial Infarction and Nonobstructive Coronary Arteries,” Circulation 131, no. 10 (March 2015): 861–870, 10.1161/CIRCULATIONAHA.114.011201. [DOI] [PubMed] [Google Scholar]
- 2. Barr P. R., Harrison W., Smyth D., Flynn C., Lee M., and Kerr A. J., “Myocardial Infarction Without Obstructive Coronary Artery Disease Is Not a Benign Condition (ANZACS‐QI 10),” Heart, Lung & Circulation 27, no. 2 (2018): 165–174, 10.1016/j.hlc.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 3. Safdar B., Spatz E. S., Dreyer R. P., et al., “Presentation, Clinical Profile, and Prognosis of Young Patients With Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): Results From the VIRGO Study,” Journal of the American Heart Association 7, no. 13 (2018): 1–22, 10.1161/JAHA.118.009174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lindahl B., Baron T., Erlinge D., et al., “Medical Therapy for Secondary Prevention and Long‐Term Outcome in Patients With Myocardial Infarction With Nonobstructive Coronary Artery Disease,” Circulation 135, no. 16 (April 2017): 1481–1489, 10.1161/CIRCULATIONAHA.116.026336. [DOI] [PubMed] [Google Scholar]
- 5. Miller R. D., Burchell H. B., and Edwards J. E., “Myocardial Infarction With and Without Acute Coronary Occlusion; A Pathologic Study,” A.M.A. Archives of Internal Medicine 88, no. 5 (1951): 597–604. [DOI] [PubMed] [Google Scholar]
- 6. Thygesen K., Alpert J. S., Jaffe A. S., et al., “Fourth Universal Definition of Myocardial Infarction (2018),” Circulation 138, no. 20 (2018): 1–34, 10.1161/cir.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 7. Dreyer R. P., Tavella R., Curtis J. P., et al., “Myocardial Infarction With Non‐Obstructive Coronary Arteries as Compared With Myocardial Infarction and Obstructive Coronary Disease: Outcomes in a Medicare Population,” European Heart Journal 41, no. 7 (February 2020): 870–878, 10.1093/eurheartj/ehz403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang F.‐Y., Huang B.‐T., Lv W.‐Y., et al., “The Prognosis of Patients With Nonobstructive Coronary Artery Disease Versus Normal Arteries Determined by Invasive Coronary Angiography or Computed Tomography Coronary Angiography: A Systematic Review,” Medicine 95, no. 11 (2016): e3117, 10.1097/MD.0000000000003117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Canton L., Fedele D., Bergamaschi L., et al., “Sex‐ and Age‐Related Differences in Outcomes of Patients With Acute Myocardial Infarction: MINOCA vs. MIOCA,” European Heart Journal: Acute Cardiovascular Care 12, no. 9 (September 2023): 604–614, 10.1093/ehjacc/zuad059. [DOI] [PubMed] [Google Scholar]
- 10. Powell‐Wiley T. M., Poirier P., Burke L. E., et al., “Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association,” Circulation 143, no. 21 (May 2021): e984–e1010, 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Piché M. E., Poirier P., Lemieux I., and Després J. P., “Overview of Epidemiology and Contribution of Obesity and Body Fat Distribution to Cardiovascular Disease: An Update,” Progress in Cardiovascular Diseases 61, no. 2 (July/August 2018): 103–113, 10.1016/j.pcad.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 12. Zhang C., Rexrode K. M., van Dam R. M., Li T. Y., and Hu F. B., “Abdominal Obesity and the Risk of All‐Cause, Cardiovascular, and Cancer Mortality: Sixteen Years of Follow‐Up in US Women,” Circulation 117, no. 13 (2008): 1658–1667, 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 13. Collaborators G. B. D. O., Afshin A., Forouzanfar M. H., et al., “Health Effects of Overweight and Obesity in 195 Countries Over 25 Years,” New England Journal of Medicine 377, no. 1 (July 2017): 13–27, 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomsen M. and Nordestgaard B. G., “Myocardial Infarction and Ischemic Heart Disease in Overweight and Obesity With and Without Metabolic Syndrome,” JAMA Internal Medicine 174, no. 1 (January 2014): 15–22, 10.1001/jamainternmed.2013.10522. [DOI] [PubMed] [Google Scholar]
- 15. Akyea R. K., Ntaios G., and Doehner W., “Obesity, Metabolic Health and Clinical Outcomes After Incident Cardiovascular Disease: A Nationwide Population‐Based Cohort Study,” Journal of Cachexia, Sarcopenia and Muscle 14 (2023): 2653–2662, 10.1002/jcsm.13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lavie C. J., Milani R. V., and Ventura H. O., “Obesity and Cardiovascular Disease,” Journal of the American College of Cardiology 53, no. 21 (May 2009): 1925–1932, 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 17. Zheng J., Hu Y., Xu H., et al., “Normal‐Weight Visceral Obesity Promotes a Higher 10‐Year Atherosclerotic Cardiovascular Disease Risk in Patients With Type 2 Diabetes Mellitus: A Multicenter Study in China,” Cardiovascular Diabetology 22, no. 1 (June 2023): 137, 10.1186/s12933-023-01876-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown O. I., Drozd M., McGowan H., et al., “Relationship Among Diabetes, Obesity, and Cardiovascular Disease Phenotypes: A UK Biobank Cohort Study,” Diabetes Care 46, no. 8 (August 2023): 1531–1540, 10.2337/dc23-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yokoyama H., Tomita H., Honda S., et al., “Effect of Low Body Mass Index on the Clinical Outcomes of Japanese Patients With Acute Myocardial Infarction: Results From the Prospective Japan Acute Myocardial Infarction Registry (JAMIR),” Circulation Journal 86, no. 4 (March 2022): 632–639, 10.1253/circj.CJ-21-0705. [DOI] [PubMed] [Google Scholar]
- 20. Bray G. A., Heisel W. E., Afshin A., et al., “The Science of Obesity Management: An Endocrine Society Scientific Statement,” Endocrine Reviews 39, no. 2 (April 2018): 79–132, 10.1210/er.2017-00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abdu F. A., Mohammed A.‐Q., Liu L., et al., “Metabolic Syndrome and the Risk of Adverse Cardiovascular Events in Patients With Myocardial Infarction With Non‐Obstructive Coronary Arteries,” Nutrition, Metabolism, and Cardiovascular Diseases 32, no. 3 (2022): 666–674, 10.1016/j.numecd.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 22. Paolisso P., Foà A., Bergamaschi L., et al., “Impact of Admission Hyperglycemia on Short and Long‐Term Prognosis in Acute Myocardial Infarction: MINOCA Versus MIOCA,” Cardiovascular Diabetology 20, no. 1 (September 2021): 192, 10.1186/s12933-021-01384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Després J.‐P. and Lemieux I., “Abdominal Obesity and Metabolic Syndrome,” Nature 444, no. 7121 (2006): 881–887. [DOI] [PubMed] [Google Scholar]
- 24. Ishii M., Kaikita K., Sakamoto K., et al., “Characteristics and In‐Hospital Mortality of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease in Super‐Aging Society,” International Journal of Cardiology 301 (February 2020): 108–113, 10.1016/j.ijcard.2019.09.037. [DOI] [PubMed] [Google Scholar]
- 25. Lu Y., Hajifathalian K., Ezzati M., et al., “Metabolic Mediators of the Effects of Body‐Mass Index, Overweight, and Obesity on Coronary Heart Disease and Stroke: A Pooled Analysis of 97 Prospective Cohorts With 1.8 Million Participants,” Lancet 383, no. 9921 (March 2014): 970–983, 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaudhry H., Bodair R., Mahfoud Z., et al., “Overweight and Obesity Are Associated With Better Survival in STEMI Patients With Diabetes,” Obesity 31, no. 11 (November 2023): 2834–2844, 10.1002/oby.23863. [DOI] [PubMed] [Google Scholar]
- 27. Lavie C. J., Alpert M. A., Arena R., Mehra M. R., Milani R. V., and Ventura H. O., “Impact of Obesity and the Obesity Paradox on Prevalence and Prognosis in Heart Failure,” JACC: Heart Failure 1, no. 2 (April 2013): 93–102, 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 28. Kang W. Y., Jeong M. H., Ahn Y. K., et al., “Are Patients With Angiographically Near‐Normal Coronary Arteries Who Present as Acute Myocardial Infarction Actually Safe?,” International Journal of Cardiology 146, no. 2 (2011): 207–212, 10.1016/j.ijcard.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 29. Nordenskjöld A. M., Lagerqvist B., Baron T., et al., “Reinfarction in Patients With Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): Coronary Findings and Prognosis,” American Journal of Medicine 132, no. 3 (March 2019): 335–346, 10.1016/j.amjmed.2018.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data can be obtained by contacting the corresponding author. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.


