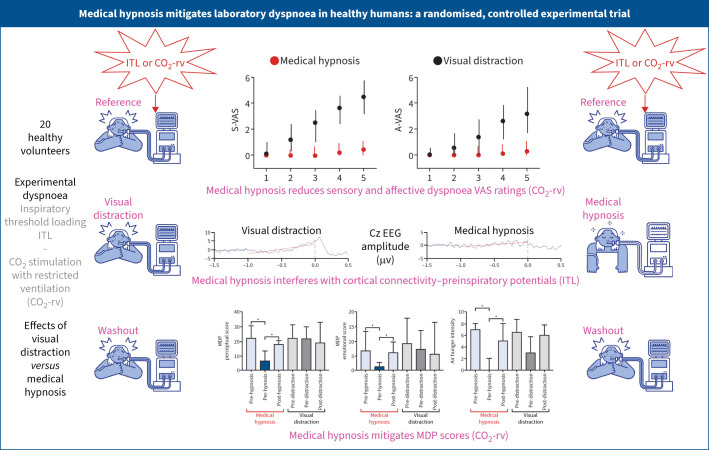
Graphical abstract
Summary of the study. ITL: inspiratory threshold loading; CO2-rv: carbon dioxide stimulation with restriction of the reflex ventilatory response; VAS: visual analogue scale; S-VAS: sensory dyspnoea VAS; A-VAS: affective dyspnoea VAS; EEG: electroencephalogram; MDP: Multidimensional Dyspnea Profile. *: p<0.05.
Abstract
Question
Dyspnoea persisting despite treatments of underlying causes requires symptomatic approaches. Medical hypnosis could provide relief without the untoward effects of pharmacological approaches. We addressed this question through experimentally induced dyspnoea in healthy humans (inspiratory threshold loading (excessive inspiratory effort) and carbon dioxide stimulation (air hunger)).
Material and methods
20 volunteers (10 women, aged 21–40 years) were studied on four separate days. The order of the visits was randomised in two steps: firstly, the “inspiratory threshold loading first” versus “carbon dioxide first” group (n=10 in each group); secondly, the “medical hypnosis first” versus “visual distraction first” subgroup (n=5 in each subgroup). Each visit comprised three 5-min periods (reference, intervention, washout) during which participants used visual analogue scales (VAS) to rate the sensory and affective dimensions of dyspnoea, and after which they completed the Multidimensional Dyspnea Profile.
Results
Medical hypnosis reduced both dimensions of dyspnoea significantly more than visual distraction (inspiratory threshold loading: sensory reduction after 5 min 34% of full VAS versus 8% (p=0.0042), affective reduction 17.6% versus 2.4% (p=0.044); carbon dioxide: sensory reduction after 5 min 36.9% versus 3% (p=0.0015), affective reduction 29.1% versus 8.7% (p=0.0023)). The Multidimensional Dyspnea Profile showed more marked sensory effects during inspiratory threshold loading and more marked affective effects during carbon dioxide stimulation.
Answer to the question
Medical hypnosis was more effective than visual distraction at attenuating the sensory and affective dimensions of experimentally induced dyspnoea. This provides a strong rationale for clinical studies of hypnosis in persistent dyspnoea patients.
Shareable abstract
Medical hypnosis is capable of spectacularly attenuating the affective and sensory components of dyspnoea by interfering with both breathing drive and cortical processing. This provides a solid experimental basis for clinical studies. https://bit.ly/3R5F6jn
Introduction
Dyspnoea is among the most distressing human experiences, even more so than pain, because of the fear of dying inherent to being unable to breathe. Its persistence despite treatments aimed at correcting the underlying causes is common, due to respiratory system damages that are frequently irreversible. The absence of a systematic therapeutic response to persistent dyspnoea [1], in contrast with pain, generates a feeling of helplessness in patients and caregivers, which is exacerbated by the condition being generally underrecognised by those not affected [2, 3]. Providing therapeutic approaches capable of improving persistent dyspnoea is therefore a central medical duty, anchored to compelling moral obligations [4]. Opiates can be effective in this indication, but treatments without their side-effects are needed. Several such approaches, pharmacological or otherwise, have been investigated, including furosemide or menthol inhalation [5, 6], trigeminal stimulation with portable fans [7, 8], musical stimulation [8], cognitive behavioural therapies [9], mindfulness [10] and virtual reality based therapy [11].
Medical hypnosis, described as a “modified state of consciousness resulting from a change in baseline mental activity after an induction procedure and typically experienced at the subjective level as an increase in absorption, focused attention, disattention to extraneous stimuli and a reduction in spontaneous thought” [12], and defined, in simpler terms, as “a state of consciousness involving focused attention and reduced peripheral awareness characterized by an enhanced capacity for response to suggestion” [13], could also be proposed. Indeed, it can alleviate the sensory and affective dimensions of pain [14], most likely through functional changes in brain areas involved in cognitive pain processing [15]. Since pain and dyspnoea share neural pathways within the central nervous system [16, 17], hypnosis could also have positive effects on dyspnoea. A preliminary study in patients with COPD [18] showed it to be more effective than a control intervention at reducing anxiety and slowing breathing frequency, but not at reducing a unidimensional measure of dyspnoea (Borg scale) [18].
Interpreting the effects of dyspnoea interventions in a clinical context is difficult because of multidimensionality and multifactoriality, hence the interest in laboratory dyspnoea experiments. There are currently no data regarding medical hypnosis in this setting. We therefore designed the present study to test the hypothesis that medical hypnosis would alleviate the affective and sensory dimensions of acute experimental dyspnoea of both the “air hunger” and “sense of excessive breathing effort” types. In addition, we hypothesised that hypnosis effects would be more marked on dyspnoea's affective dimension and thus more marked for experimental air hunger, being more affectively distressing than excessive breathing effort [19].
To test these hypotheses, we conducted a proof-of-concept study of medical hypnosis in experimental dyspnoea, with visual distraction as a comparator and with subjective and objective assessments of efficacy.
Methods
Details on specific techniques are provided in the supplementary material.
Ethics
This was a randomised, crossover, open-label, controlled study conducted at a single site in Paris, France (clinicaltrials.gov identifier NCT02792738). The study protocol was approved by the appropriate ethical body (Comité de Protection des Personnes Ile-de-France VI, Paris, France). Participants received detailed information, provided written consent before enrolment, and received a financial incentive after study completion.
Participants
Healthy individuals aged >18 years were enrolled. They had no prior experience of physiology experiments or of hypnosis and their hypnotic susceptibility was unknown (noninclusion criteria are listed in the supplementary material). During the 48 h preceding the experiments, the participants were asked to refrain from consuming analgesic and anti-inflammatory medications, alcohol, caffeine or any psychotropic substances, and to avoid sleep deprivation.
Study protocol
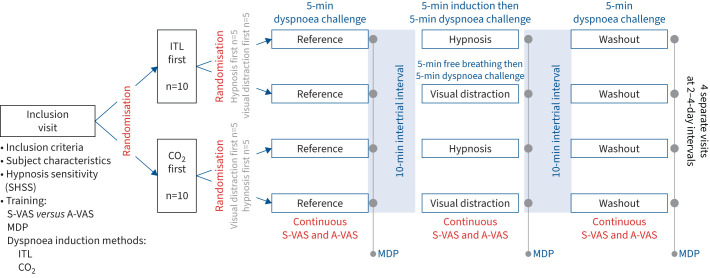
The study comprised an inclusion visit (visit 1) and four subsequent visits (visits 2–5) separated by 2–4 days (figure 1). During visit 1, the participants’ hypnotic susceptibility was tested using the Stanford Hypnosis Sensitivity Scale. The notion of sensory versus affective dimensions of dyspnoea was explained using a music analogy (sensory dimension: volume of the music played; affective dimension: agreement/disagreement evoked by the music: “if you do not like a [piece of] music, it does not have to be loud to be disagreeable”) and participants were trained to answer the Multidimensional Dyspnea Profile (MDP) [20, 21]. Finally, participants were familiarised with the dyspnoea-inducing procedures, namely inspiratory threshold loading (ITL) and carbon dioxide (CO2) stimulation with restricted ventilation (CO2-rv). During ITL and CO2-rv familiarisation, we aimed to obtain visual analogue scale (VAS) ratings of the affective or the sensory dimension of dyspnoea of ∼50% of the full scale (described later). During the four subsequent visits, the participants underwent three 5-min dyspnoea inductions separated by 10-min intervals according to a “reference”, “intervention” and “washout” sequence (“washout” as in “recovery”, not as in “before next session”) (figure 1). The order of the visits was randomised in two steps, firstly “ITL first then CO2-rv” versus “CO2-rv first then ITL” (n=10 in each group); secondly “medical hypnosis first then visual distraction” versus “visual distraction first then hypnosis” subgroups (n=5 in each subgroup). Randomisations were achieved using Microsoft Excel.
FIGURE 1.
Diagrammatic summary of the experimental protocol. SHSS: Stanford Hypnosis Sensitivity Scale; VAS: visual analogue scale; S-VAS: sensory dyspnoea VAS; A-VAS: affective dyspnoea VAS; MDP: Multidimensional Dyspnea Profile; ITL: inspiratory threshold loading (predominantly induces “excessive inspiratory effort”); CO2: carbon dioxide stimulation with restriction of the reflex ventilatory response (predominantly induces “air hunger”).
Procedures
Induction of experimental dyspnoea
During the experiments, participants were seated comfortably in a semi-reclined position with their arms and heads supported (supplementary material).
Inspiratory threshold loading
To elicit dyspnoea predominantly of the excessive inspiratory effort type, the participants breathed through a spring-loaded threshold valve (PowerBreathe, UK), adjusted, during familiarisation at visit 1, to produce VAS ratings of either the affective or the sensory dimension of dyspnoea of ∼50% of the full scale (first reached).
Carbon dioxide stimulation with restricted ventilation
To elicit dyspnoea predominantly of the air hunger type we used CO2-rv (procedure adapted from [19]). The participants were placed under mechanical ventilation via a face mask, with a ventilator set in volume-controlled mode and aligned on the participants' resting breathing pattern to provide full breathing comfort. During familiarisation at visit 1, 99.9% CO2 was progressively instilled into the inspiratory limb of the breathing circuit to induce air hunger, until a 50% VAS rating of either the affective or the sensory dimension of dyspnoea was reached.
Dyspnoea-relieving interventions
Medical hypnosis
Hypnosis was conducted using a standardised script (supplementary material) by physicians certified in medical hypnosis (C. Arveiller-Carvallo, A. Brion, C. Morélot-Panzini). The induction procedure (derived from [22]) involved relaxation (the script including “your breathing is gentle and is slowing down, you can feel the air come into your mouth and go right to the depths of your lungs”), binding between the participant and the experimenter, and the establishment of a hypnotic state (considered present when roving eye movements could be observed and the subjects indicated that they felt having reached their “safe place” through a pre-arranged finger movement). This lasted for ∼5 min, after which the experimental dyspnoea challenge started for 5 min, the hypnotic state being maintained throughout the exposure to the dyspnoea-inducing stimulus; the instruction to “come back” being given simultaneously with stimulus removal. Of note, the participants were responsive during the hypnotic phase, as evidenced by their continuous rating of dyspnoea (described later). Post hoc electroencephalographic (EEG) readings confirmed the absence of sleep-related figures.
Visual distraction
Visual distraction consisted of watching an emotionally neutral extract of an animal documentary (March of the Penguins, Luc Jacquet, France, 2005). To mirror the medical hypnosis procedure that included an induction period before the dyspnoea challenge, 5 min of viewing during free breathing preceded the 5 min of viewing during the dyspnoea challenge.
Psychophysiological measurements
Real-time bidimensional evaluation of dyspnoea
The participants used two separate 10-cm electronic VAS (slide control response meters; AD Instruments, Castle Hill, Australia) for sensory rating (S-VAS) and affective rating (A-VAS) (detailed rating instructions are presented in the supplementary material). They were instructed to move the S-VAS and A-VAS cursors as needed to record any change, without any instruction regarding in which order to do so. If they did not move the cursors for 1 min, the experimenter prompted them to provide a rating through a pre-arranged arm touch. VAS values were averaged minute by minute.
Post-hoc multidimensional evaluation of dyspnoea
The participants filled out the MDP (MDP items are presented in the supplementary material) at the end of each dyspnoea induction session (i.e. three times at each of visits 2–5; figure 1). They focused on the last 30 s spent under experimental dyspnoea, and MDP data were analysed in terms of an “immediate perception response” and an “emotional response domain”, in line with pain conceptual models.
Physiological measurements
During each procedure (ITL or CO2-rv), participants breathed either through a mouthpiece (ITL) or a face mask (CO2-rv). The following variables were measured or calculated (technical details are presented in the supplementary material): airway opening pressure (Pao), ventilatory flow, tidal volume (VT), inspiratory time (tI), expiratory time (tE), total time (tTOT), breathing frequency, mean inspiratory flow (VT/tI), duty cycle (tI/tTOT), Pao pressure–time product (PTP), end-tidal CO2 partial pressure in the expired gas (PETCO2), heart frequency and galvanic skin response.
Electroencephalographic recordings
EEG acquisition
EEG activity was recorded with eight cap-mounted active electrodes (actiCAP; Brain Products, Germany), placed in Cz, FCz (reference), Fz, AFz (ground), FP1, FP2, A1 and A2. Electrode impedance was maintained below 5 kΩ. Data acquisition used a V-Amp amplifier (Brain Products). Signals were filtered analogically with a low-pass value of 0.05 Hz, a high-pass value of 5 Hz and a band-stop filter centred on 50 Hz. EEG signals were amplified and digitised at 256 Hz and then processed as follows.
EEG processing
Firstly, to identify nonspecific changes in brain cortical connectivity, we followed a previously described Riemannian matrix covariance analysis and applied an in-house classification algorithm to discriminate experimental conditions according to brain activity using a semi-supervised approach [23–27]) (detailed EEG processing is described in the supplementary material). Secondly, we used a previously described event-related approach to identify respiratory-specific pre-inspiratory potentials [26, 28–30] (refer to figure 1 in [30], and the supplementary material).
Outcomes
The primary study outcome was the intensity of breathing discomfort (A-VAS). The sensory intensity of dyspnoea (S-VAS) was a co-primary outcome. Secondary outcomes included MDP, ventilatory variables, heart frequency and galvanic skin response.
Statistical analysis
Statistical analyses were conducted with Matlab version 9.7.0.1261785. Data are described as median and interquartile range (IQR). p<0.05 was considered statistically significant. S-VAS and A-VAS differences (ΔVAS) between hypnosis and visual distraction were analysed minute-by-minute using repeated Wilcoxon paired tests with Holm's adjustment for multiplicity. This approach was corroborated by implementing linear mixed models fitted using the maximum likelihood method, also with Holm's adjustment of the fixed slopes and intercepts p-values. Physiological data were compared using a two-way ANOVA with a time factor (reference, intervention, washout) and a condition factor (hypnosis, visual distraction). Correlations between differences in VAS ratings and Stanford Hypnosis Sensitivity Scale were studied using Spearman's test. Of note, in the absence of prior data that would have allowed a sample size estimate, a convenience sample of 20 participants (10 women and 10 men) was planned. A detailed description of the statistical analysis is presented in the supplementary material).
Results
Study participants
20 healthy adults (10 women, 10 men; age 21–40 years) completed all five vitsits as planned.
Hypnosis sensitivity
Median (IQR) Stanford Hypnosis Susceptibility Scale score was 5 (3–8) (≥8=high susceptibility: 8 cases; ≤4=limited susceptibility: 8 cases). Based on behavioural cues we considered that a hypnotic state was achieved in all participants.
Responses to inspiratory threshold loading
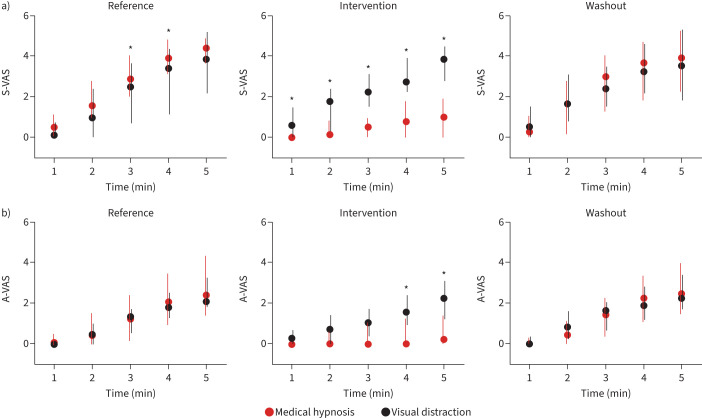
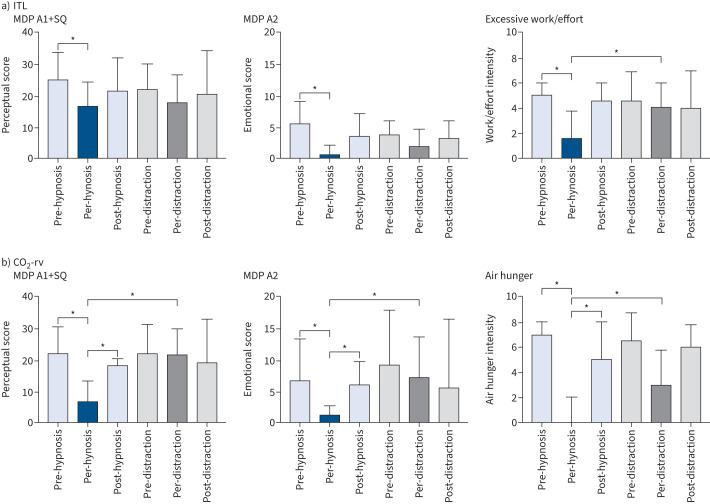
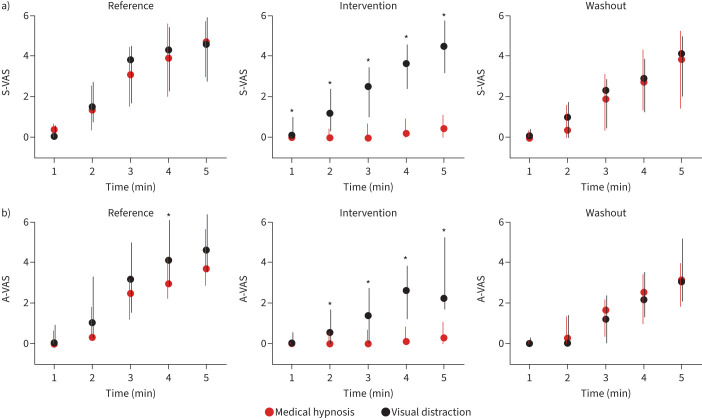
During medical hypnosis, breathing frequency decreased significantly, and PETCO2 increased significantly for the last 4 min of the dyspnoea challenge (supplementary figure S2) suggesting a reduced neural drive to breathe (in line with decreases in peak Pao and mean inspiratory flow (VT/tI)) (supplementary figure S3). There was no change in VT, PTP or tI/tTOT (supplementary figures S2 and S3). During visual distraction, no significant changes in breathing pattern were observed (supplementary figures S2 and S3). ITL consistently induced dyspnoea of the excessive inspiratory effort type. During the reference and washout periods, S-VAS and A-VAS ratings increased similarly over the 5 min of loading (figure 2, supplementary figure S4). This dynamic was not affected by visual distraction (figure 2, supplementary figure S4). In contrast, medical hypnosis significantly mitigated sensory ratings (from the first minute onwards) and affective ratings (at the fourth and fifth minutes) (figure 2, table 1; supplementary figure S4). There was no significant correlation between dyspnoea attenuation and the Stanford Hypnosis Sensitivity Scale score. The effects of hypnosis did not vary between hypnotherapists. Medical hypnosis significantly decreased the MDP perceptual score (A1+SQ, p=0.009) and the MDP affective score (A2, p=0.002), both returning to pre-hypnosis levels during washout (figure 3). MDP changes did not reach statistical significance during visual distraction. There was a significant reduction in the “work/effort” descriptor during medical hypnosis (p=0.0024), but not during visual distraction, hence a significant hypnosis–visual distraction difference (p=0.0034) (figure 3, supplementary table S1).
FIGURE 2.
Visual analogue scale (VAS) dyspnoea ratings in response to 5 min of inspiratory threshold loading. a) Sensory VAS (S-VAS); b) affective VAS (A-VAS). *: p<0.05 between medical hypnosis and visual distraction.
TABLE 1.
Comparison of the reductions in dyspnoea ratings by visual distraction and medical hypnosis at the fifth minute of the dyspnoea challenges, showing agreement between the nonparametric and the mixed-model statistical approaches
| Visual distraction | Medical hypnosis | p-value visual distraction minus hypnosis |
||
|---|---|---|---|---|
| Nonparametric model | Linear mixed model | |||
| Sensory VAS | ||||
| ITL | −0.08 (−1.12– −0.26) | −3.40 (−4.84– −0.85) | 0.0042 | 5.6×10−7 |
| CO2-rv | −0.30 (−1.45– −0.74) | −3.69 (−5.14– −1.74) | 0.0015 | 1.6×10−11 |
| Affective VAS | ||||
| ITL | −0.24 (−0.72– −0.32) | −1.76 (−3.30– −0.08) | 0.044 | 5×10−7 |
| CO2-rv | −0.87 (−2.03– −0.18) | −2.91 (−4.46– −2.37) | 0.0023 | 1.6×10−10 |
VAS: visual analogue scale; ITL: inspiratory threshold loading; CO2-rv: carbon dioxide stimulation with restricted ventilation.
FIGURE 3.
Multidimensional Dyspnea Profile (MDP) post hoc evaluation of dyspnoea in response to a) inspiratory threshold loading (ITL) and b) carbon dioxide stimulation with ventilatory restriction. The participants were asked to focus on the last 30 s of the immediately preceding condition. A1: respiratory discomfort; A1+SQ: perceptual score; A2: emotional score. In each of the three panels, the first three bars correspond to the hypnosis intervention, and the three subsequent bars correspond to the visual distraction intervention. *: p<0.05.
Responses to CO2-rv
During both medical hypnosis and visual distraction, no statistically significant changes were noted in breathing frequency, VT, peak Pao, PETCO2, PTP, VT/tI, or tI/tTOT. CO2 stimulation consistently induced dyspnoea of the air-hunger type. During the reference and washout periods, sensory and affective dyspnoea ratings similarly increased over the 5 min of loading (figure 4, supplementary figure S5); however, this showed a tendency to habituation (figure 4). This dynamic was not affected by visual distraction (figure 4, supplementary figure S5). In contrast, medical hypnosis significantly mitigated sensory ratings and affective ratings from the first minute onwards (figure 4, table 1, supplementary figure S5). There was no influence of suggestibility or of the hypnotherapist. Medical hypnosis significantly decreased the MDP perceptual score (A1+SQ) (p<0.0001) and the MDP affective score (A2) (p=0.0007); both scores returning to pre-hypnosis levels during washout (figure 3). MDP changes did not reach statistical significance during visual distraction. There was a significant reduction in the “air hunger” rating during medical hypnosis (p<0.0001) but not during visual distraction; hence, a significant hypnosis–visual distraction difference (p=0.0005) (figure 3, supplementary table S1). The “anxiety” rating decreased significantly following hypnosis (p<0.0001), also with a significant hypnosis–visual distraction difference (p=0.0005). Overall, the effects of medical hypnosis on dyspnoea appeared more marked during CO2 stimulation than during ITL.
FIGURE 4.
Visual analogue scale (VAS) dyspnoea ratings in response to 5 min of carbon dioxide stimulation with ventilatory restriction. a) Sensory VAS (S-VAS); b) affective VAS (A-VAS). Data are presented as median (interquartile range). *: p<0.05 between medical hypnosis and visual distraction.
Other physiological variables
Cardiac frequency decreased significantly with time during both dyspnoea-relieving interventions for both dyspnoea challenges. Galvanic skin response did not vary during hypnosis for ITL (p=0.73, valid signal in only nine participants), but decreased significantly for CO2-rv-induced dyspnoea (p<0.0001, valid signal in only six participants).
EEG data
EEG data were technically interpretable in 15 participants during ITL and in 14 during CO2-rv. Connectivity changes occurred between the reference and the intervention period in the majority of cases (ITL: 12 participants during visual distraction, 11 during medical hypnosis; CO2-rv: 11 and 10, respectively), indicating that both interventions modified brain state in some way. In contrast, no changes were detected between intervention and washout, suggesting long-lasting effects. Respiratory specific pre-inspiratory potentials were not significantly affected quantitatively, but appeared attenuated in amplitude during medical hypnosis in half the participants (supplementary material).
Discussion
This study showed that medical hypnosis can alleviate the sensory and affective dimensions of experimentally induced dyspnoea in healthy humans. This was true in response to two challenges known to induce distinct dyspnoeic sensory modalities (ITL/sense of excessive inspiratory effort versus CO2-rv/air hunger) and distinct affective responses [19], and to involve different brain networks. The effects of medical hypnosis were more marked for air hunger, with a relief magnitude comparable to that obtained with morphine in a similar setting [31]. Attentional distraction, otherwise reported to have mitigating effects on experimental dyspnoea [32, 33], was not associated to significant dyspnoea relief. In contrast to experimental pain studies [34], there was no relationship between medical hypnosis effects and individual hypnotic sensitivity. Of note, the EEG recordings performed in our participants did not evidence sleep-related figures during medical hypnosis. They showed that both visual distraction and medical hypnosis were associated with nonspecific brain connectivity changes, indicating that both interventions had an effect at the cortical level but without providing insight regarding the differential impact of the two interventions on dyspnoea. EEG recordings failed to demonstrate a statistically significant difference regarding the frequency of respiratory specific pre-inspiratory potentials between visual distraction and medical hypnosis, even though these potentials appeared attenuated during medical hypnosis (supplementary material). One possible explanation for these EEG results could lie in medical hypnosis acting at a brain level (e.g. limbic cortex) not easily accessible to scalp recordings.
Putative mechanisms
Symptomatic interventions that relieve dyspnoea can do so by decreasing the neural drive to breathe (corollary discharge theory [35, 36]) or by altering the central processing of respiratory sensations [8, 36, 37]. In our subjects during ITL there was evidence of breathing drive reduction under medical hypnosis (decreased breathing frequency, increased PETCO2 (supplementary figure S2); decreased VT/tI (supplementary figure S3)). Thus, hypnosis could have acted on the cortical component of the neural drive to breathe associated with ITL [29] and causatively related to clinical dyspnoea in various settings [26, 27, 38]. This was supported in some participants by attenuation of pre-inspiratory potentials. During CO2-rv, we found no indication of breathing drive reduction during medical hypnosis (supplementary figures S2 and S3), yet dyspnoea relief was more marked than during ITL. This points at a “processing” type of effect, reminiscent of the effects of medical hypnosis on pain [39]. This is consistent with neuroimaging studies showing that hypnosis modulates the activity and connectivity of a large brain network [40–44] that comprises regions involved in the pathogenesis of dyspnoea, including the anterior cingulate cortex, the right insula and the supplementary motor area, as well as many others (reviewed in [45]). Our EEG results support the role of hypnosis-related brain connectivity changes, but specific brain imaging studies will be necessary to precisely describe the involved networks and possibly devise targeted interventions [46].
Dyspnoea is characteristically associated with anxiety. Yet, therapeutic approaches that relieve anxiety can help dyspnoeic patients independently of their effect on dyspnoea itself, as shown for hypnosis in patients with COPD [18]. The known anxiety-relieving effects of hypnosis [47] were apparent in our study, in the form of a reduction in the corresponding MDP ratings that was most pronounced during CO2-rv, described as more anxiogenic than inspiratory loading [19]. This observation aligns with the attenuation of the galvanic skin response observed under hypnosis during CO2-rv, but not during ITL.
Strengths, limitations and questions for future research
A strength of our study was its controlled, crossover design, but the open-label aspect meant that cognitive bias favouring hypnosis over visual distraction was possible. To minimise this risk, participants were informed that the study aimed “to compare two interventions possibly relieving dyspnoea” rather than “to evaluate the effectiveness of hypnosis”. Additionally, three hypnotherapists were responsible for the hypnosis intervention to further reduce cognitive bias. Other strengths of the study include the incorporation of two models of experimental dyspnoea, the coherence of psychophysiological outcomes and physiological ones, and a dual statistical approach showing consistent results. The main limitation of the study lies in the imperfect representation of clinical dyspnoea by experimental dyspnoea models, with participants knowing the dyspnoea challenges to be time-limited and retaining control over their situation. Another limitation of our study relates to the brevity of the dyspnoea challenges that we used, which did not allow dyspnoea ratings to stabilise over time. In addition, we needed to train our subjects before the actual experiments, and observed that after training we were unable to reproduce the dyspnoea levels initially observed, hence a possible floor effect due to relatively modest dyspnoea ratings at the beginning of the dyspnoea challenge. Of note, we did not characterise our participants regarding trait or state anxiety, which could have modulated the effects of hypnosis. Finally, the size of the study population implies caution, particularly regarding secondary outcomes. However, the magnitude of the effect of hypnosis on VAS ratings exceeded minimal clinically important differences [48–50], suggesting that clinical benefits could be obtained in patients with persistent dyspnoea. However, effect duration, tachyphylaxis or the level of hypnosis expertise required to obtain an effect cannot be predicted at this stage. These elements will condition practical applicability, and will determine whether particular strategies should be explored, from highly sophisticated ones involving cortical sensitisation [46] to simpler ones such as self-hypnosis, which has proven useful in other situations [51]. It is thus necessary design studies of medical hypnosis in patients with persistent dyspnoea in the real world, taking care to use multidimensional outcomes [52]. To define optimal indications, such studies will need to explore the role of individual hypnotic susceptibility and the importance of protocols.
Conclusions
In conclusion, we believe that our results provide a solid proof-of-concept to pursue the study of medical hypnosis to manage persistent dyspnoea, noting that empirical clinical studies are appearing [18, 53]. If effective, medical hypnosis would have several advantages, including fast implementation, rapid effects, low cost, and lack of significant risks (see review in [54]).
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00485-2024.SUPPLEMENT (1.4MB, pdf)
Shareable PDF
Acknowledgements
Andrew Lane (Lane Medical Writing, Lyon, France) provided professional medical writing assistance in the preparation of the manuscript in accordance with the European Medical Writers Association guidelines and Good Publication Practice. He was funded by research unit UMRS1158, Inserm-Sorbonne Université, Paris, France.
Footnotes
This study is registered at www.clinicaltrials.gov with identifier number NCT02792738.
Ethics statement: The study protocol was approved by the appropriate ethical and regulatory bodies according to French law (Comité de Protection des Personnes Ile-de-France VI, Paris, France and Agence Nationale de Sécurité du Médicament), and the study was conducted according to the Declaration of Helsinki. Detailed information was provided and written consent was obtained before enrolment. Each participant received a financial incentive.
Author contributions: C. Morélot-Panzini, C. Arveiller-Carvallo, S. Lavault, A. Brion, M-C. Niérat and T. Similowski: conception or design of the work; acquisition, analysis, or interpretation of data; drafting or revising critically the manuscript for intellectual content; final approval; accountable for all aspects. I. Rivals, N. Wattiez, L. Serresse and C. Straus: acquisition, analysis or interpretation of data; drafting or revising critically for intellectual content; final approval; accountable for all aspects.
This article has an editorial commentary: https://doi.org/10.1183/13993003.01141-2024
Conflict of interest: C. Morélot-Panzini reports grants from Fondation du Souffle and lecture honoraria from Chiesi, outside the submitted work.
Conflict of interest: L. Serresse reports lecture honoraria from Chiesi and travel support from SOS Oxygene, outside the submitted work.
Conflict of interest: T. Similowski reports consulting fees from AstraZeneca, Chiesi, KPL Consulting, Lungpacer Inc. and OSO-AI; lecture honoraria from Chiesi and Vitalaire; stock or stock options from AUSTRAL Dx and HEPHAI; and the following patents: WO2008006963A3, WO2012004534A1, WO2013164462A1; outside the submitted work.
Conflict of interest: The remaining authors have no potential conflicts of interest to disclose.
Support statement: The study was solely academically funded, and supported by a grant from Fonds de Recherche en Santé Respiratoire and Fondation du Souffle Paris, France. It was also supported by a grant Legs Poix from the Chancellerie de l'Université de Paris and by the Association pour le Développement et l'Organisation de la Recherche en Pneumologie, Paris, France. Funding information for this article has been deposited with the Crossref Funder Registry.
Data availability
Data will be shared with researchers upon reasonable request.
References
- 1.Morélot-Panzini C, Adler D, Aguilaniu B, et al. . Breathlessness despite optimal pathophysiological treatment: on the relevance of being chronic. Eur Respir J 2017; 50: 1701159. doi: 10.1183/13993003.01159-2017 [DOI] [PubMed] [Google Scholar]
- 2.Carel H. Invisible suffering: the experience of breathlessness. In: Škof L, Berndtson P, eds. Atmospheres of Breathing. Albany, NY, State University of New York Press, 2018. Available from: www.ncbi.nlm.nih.gov/books/NBK535570/. [PubMed] [Google Scholar]
- 3.Serresse L, Guerder A, Dedonder J, et al. . ‘You can't feel what we feel': multifaceted dyspnoea invisibility in advanced chronic obstructive pulmonary disease examined through interpretative phenomenological analysis. Palliat Med 2022; 36: 1364–1373. doi: 10.1177/02692163221118198 [DOI] [PubMed] [Google Scholar]
- 4.Bașoğlu M. Effective management of breathlessness: a review of potential human rights issues. Eur Respir J 2017; 49: 1602099. doi: 10.1183/13993003.02099-2016 [DOI] [PubMed] [Google Scholar]
- 5.Grogono JC, Butler C, Izadi H, et al. . Inhaled furosemide for relief of air hunger versus sense of breathing effort: a randomized controlled trial. Respir Res 2018; 19: 181. doi: 10.1186/s12931-018-0886-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanezaki M, Terada K, Ebihara S. Effect of olfactory stimulation by l-menthol on laboratory-induced dyspnea in COPD. Chest 2020; 157: 1455–1465. doi: 10.1016/j.chest.2019.12.028 [DOI] [PubMed] [Google Scholar]
- 7.Luckett T, Phillips J, Johnson MJ, et al. . Contributions of a hand-held fan to self-management of chronic breathlessness. Eur Respir J 2017; 50: 1700262. doi: 10.1183/13993003.00262-2017 [DOI] [PubMed] [Google Scholar]
- 8.Bureau C, Niérat MC, Decavèle M, et al. . Sensory interventions to relieve dyspnoea in critically ill mechanically ventilated patients. Eur Respir J 2024; 63: 2202215. doi: 10.1183/13993003.02215-2022 [DOI] [PubMed] [Google Scholar]
- 9.Farver-Vestergaard I, O'Toole MS, O'Connor M, et al. . Mindfulness-based cognitive therapy in COPD: a cluster randomised controlled trial. Eur Respir J 2018; 51: 1702082. doi: 10.1183/13993003.02082-2017 [DOI] [PubMed] [Google Scholar]
- 10.Tan SB, Liam CK, Pang YK, et al. . The effect of 20-minute mindful breathing on the rapid reduction of dyspnea at rest in patients with lung diseases: a randomized controlled trial. J Pain Symptom Manage 2019; 57: 802–808. doi: 10.1016/j.jpainsymman.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 11.Betka S, Kannape OA, Fasola J, et al. . Virtual reality intervention alleviates dyspnoea in patients recovering from COVID-19 pneumonia. ERJ Open Res 2023; 9: 00570-2022. doi: 10.1183/23120541.00570-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakley DA, Halligan PW. Hypnotic suggestion and cognitive neuroscience. Trends Cogn Sci 2009; 13: 264–270. doi: 10.1016/j.tics.2009.03.004 [DOI] [PubMed] [Google Scholar]
- 13.Elkins GR, Barabasz AF, Council JR, et al. . Advancing research and practice: the revised APA division 30 definition of hypnosis. Am J Clin Hypn 2015; 57: 378–385. doi: 10.1080/00029157.2015.1011465 [DOI] [PubMed] [Google Scholar]
- 14.Thompson T, Terhune DB, Oram C, et al. . The effectiveness of hypnosis for pain relief: a systematic review and meta-analysis of 85 controlled experimental trials. Neurosci Biobehav Rev 2019; 99: 298–310. doi: 10.1016/j.neubiorev.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 15.Del Casale A, Ferracuti S, Rapinesi C, et al. . Hypnosis and pain perception: an activation likelihood estimation (ALE) meta-analysis of functional neuroimaging studies. J Physiol Paris 2015; 109: 165–172. doi: 10.1016/j.jphysparis.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 16.von Leupoldt A, Sommer T, Kegat S, et al. . Dyspnea and pain share emotion-related brain network. Neuroimage 2009; 48: 200–206. doi: 10.1016/j.neuroimage.2009.06.015 [DOI] [PubMed] [Google Scholar]
- 17.Dangers L, Laviolette L, Similowski T, et al. . Interactions between dyspnea and the brain processing of nociceptive stimuli: experimental air hunger attenuates laser-evoked brain potentials in humans. Front Physiol 2015; 6: 358. doi: 10.3389/fphys.2015.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anlló H, Herer B, Delignières A, et al. . Hypnosis for the management of anxiety and dyspnea in COPD: a randomized, sham-controlled crossover trial. Int J Chron Obstruct Pulmon Dis 2020; 15: 2609–2620. doi: 10.2147/COPD.S267019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banzett RB, Pedersen SH, Schwartzstein RM, et al. . The affective dimension of laboratory dyspnea: air hunger is more unpleasant than work/effort. Am J Respir Crit Care Med 2008; 177: 1384–1390. doi: 10.1164/rccm.200711-1675OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banzett RB, O'Donnell CR, Guilfoyle TE, et al. . Multidimensional Dyspnea Profile: an instrument for clinical and laboratory research. Eur Respir J 2015; 45: 1681–1691. doi: 10.1183/09031936.00038914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morélot-Panzini C, Gilet H, Aguilaniu B, et al. . Real-life assessment of the multidimensional nature of dyspnoea in COPD outpatients. Eur Respir J 2016; 47: 1668–1679. doi: 10.1183/13993003.01998-2015 [DOI] [PubMed] [Google Scholar]
- 22.Faymonville ME, Laureys S, Degueldre C, et al. . Neural mechanisms of antinociceptive effects of hypnosis. Anesthesiology 2000; 92: 1257–1267. doi: 10.1097/00000542-200005000-00013 [DOI] [PubMed] [Google Scholar]
- 23.Hudson AL, Navarro-Sune X, Martinerie J, et al. . Electroencephalographic detection of respiratory-related cortical activity in humans: from event-related approaches to continuous connectivity evaluation. J Neurophysiol 2016; 115: 2214–2223. doi: 10.1152/jn.01058.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarro-Sune X, Hudson AL, De Vico Fallani F, et al. . Riemannian geometry applied to detection of respiratory states from EEG signals: the basis for a brain–ventilator interface. IEEE Trans Biomed Eng 2017; 64: 1138–1148. doi: 10.1109/TBME.2016.2592820 [DOI] [PubMed] [Google Scholar]
- 25.Grosselin F, Navarro-Sune X, Raux M, et al. . CARE-rCortex: a Matlab toolbox for the analysis of cardio-respiratory-related activity in the cortex. J Neurosci Methods 2018; 308: 309–316. doi: 10.1016/j.jneumeth.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 26.Raux M, Navarro-Sune X, Wattiez N, et al. . Adjusting ventilator settings to relieve dyspnoea modifies brain activity in critically ill patients: an electroencephalogram pilot study. Sci Rep 2019; 9: 16572. doi: 10.1038/s41598-019-53152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decavèle M, Bureau C, Campion S, et al. . Interventions relieving dyspnea in intubated patients show responsiveness of the mechanical ventilation-respiratory distress observation scale. Am J Respir Crit Care Med 2023; 208: 39–48. doi: 10.1164/rccm.202301-0188OC [DOI] [PubMed] [Google Scholar]
- 28.Raux M, Ray P, Prella M, et al. . Cerebral cortex activation during experimentally induced ventilator fighting in normal humans receiving noninvasive mechanical ventilation. Anesthesiology 2007; 107: 746–755. doi: 10.1097/01.anes.0000287005.58761.e8 [DOI] [PubMed] [Google Scholar]
- 29.Raux M, Straus C, Redolfi S, et al. . Electroencephalographic evidence for pre-motor cortex activation during inspiratory loading in humans. J Physiol 2007; 578: 569–578. doi: 10.1113/jphysiol.2006.120246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taytard J, Gand C, Niérat MC, et al. . Impact of inspiratory threshold loading on brain activity and cognitive performances in healthy humans. J Appl Physiol 2022; 132: 95–105. doi: 10.1152/japplphysiol.00994.2020 [DOI] [PubMed] [Google Scholar]
- 31.Banzett RB, Adams L, O'Donnell CR, et al. . Using laboratory models to test treatment: morphine reduces dyspnea and hypercapnic ventilatory response. Am J Respir Crit Care Med 2011; 184: 920–927. doi: 10.1164/rccm.201101-0005OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabler MC, Goss CS, Freemas JA, et al. . Dyspnea is attenuated by auditory distraction via music with headphones during exercise in healthy individuals. Med Sci Sports Exerc 2022; 54: 1973–1981. doi: 10.1249/MSS.0000000000002982 [DOI] [PubMed] [Google Scholar]
- 33.von Leupoldt A, Seemann N, Gugleva T, et al. . Attentional distraction reduces the affective but not the sensory dimension of perceived dyspnea. Respir Med 2007; 101: 839–844. doi: 10.1016/j.rmed.2006.06.033 [DOI] [PubMed] [Google Scholar]
- 34.Tenenbaum SJ, Kurtz RM, Bienias JL. Hypnotic susceptibility and experimental pain reduction. Am J Clin Hypn 1990; 33: 40–49. doi: 10.1080/00029157.1990.10402899 [DOI] [PubMed] [Google Scholar]
- 35.Banzett RB, Lansing RW, Reid MB, et al. . ‘Air hunger' arising from increased PCO2 in mechanically ventilated quadriplegics. Respir Physiol 1989; 76: 53–67. doi: 10.1016/0034-5687(89)90017-0 [DOI] [PubMed] [Google Scholar]
- 36.Parshall MB, Schwartzstein RM, Adams L, et al. . An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012; 185: 435–452. doi: 10.1164/rccm.201111-2042ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Similowski T. Treat the lungs, fool the brain and appease the mind: towards holistic care of patients who suffer from chronic respiratory diseases. Eur Respir J 2018; 51: 1800316. doi: 10.1183/13993003.00316-2018 [DOI] [PubMed] [Google Scholar]
- 38.Georges M, Morawiec E, Raux M, et al. . Cortical drive to breathe in amyotrophic lateral sclerosis: a dyspnoea-worsening defence? Eur Respir J 2016; 47: 1818–1828. doi: 10.1183/13993003.01686-2015 [DOI] [PubMed] [Google Scholar]
- 39.Dahlgren LA, Kurtz RM, Strube MJ, et al. . Differential effects of hypnotic suggestion on multiple dimensions of pain. J Pain Symptom Manage 1995; 10: 464–470. doi: 10.1016/0885-3924(95)00055-4 [DOI] [PubMed] [Google Scholar]
- 40.Demertzi A, Soddu A, Faymonville ME, et al. . Hypnotic modulation of resting state fMRI default mode and extrinsic network connectivity. Prog Brain Res 2011; 193: 309–322. doi: 10.1016/B978-0-444-53839-0.00020-X [DOI] [PubMed] [Google Scholar]
- 41.Vanhaudenhuyse A, Laureys S, Faymonville ME. Neurophysiology of hypnosis. Neurophysiol Clin 2014; 44: 343–353. doi: 10.1016/j.neucli.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 42.Vanhaudenhuyse A, Boly M, Balteau E, et al. . Pain and non-pain processing during hypnosis: a thulium-YAG event-related fMRI study. Neuroimage 2009; 47: 1047–1054. doi: 10.1016/j.neuroimage.2009.05.031 [DOI] [PubMed] [Google Scholar]
- 43.Faymonville ME, Roediger L, Del Fiore G, et al. . Increased cerebral functional connectivity underlying the antinociceptive effects of hypnosis. Brain Res Cogn Brain Res 2003; 17: 255–262. doi: 10.1016/S0926-6410(03)00113-7 [DOI] [PubMed] [Google Scholar]
- 44.Jiang H, White MP, Greicius MD, et al. . Brain activity and functional connectivity associated with hypnosis. Cereb Cortex 2017; 27: 4083–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoeckel MC, Esser RW, Gamer M, et al. . Brain responses during the anticipation of dyspnea. Neural Plast 2016; 2016: 6434987. doi: 10.1155/2016/6434987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faerman A, Bishop JH, Stimpson KH, et al. . Stanford Hypnosis Integrated with Functional Connectivity-targeted Transcranial Stimulation (SHIFT): a preregistered randomized controlled trial. Nat Ment Health 2024; 2: 96–103. doi: 10.1038/s44220-023-00184-z [DOI] [Google Scholar]
- 47.Valentine KE, Milling LS, Clark LJ, et al. . The efficacy of hypnosis as a treatment for anxiety: a meta-analysis. Int J Clin Exp Hypn 2019; 67: 336–363. doi: 10.1080/00207144.2019.1613863 [DOI] [PubMed] [Google Scholar]
- 48.Ekström MP, Bornefalk H, Sköld CM, et al. . Minimal clinically important differences and feasibility of Dyspnea-12 and the Multidimensional Dyspnea Profile in cardiorespiratory disease. J Pain Symptom Manage 2020; 60: 968–975. doi: 10.1016/j.jpainsymman.2020.05.028 [DOI] [PubMed] [Google Scholar]
- 49.Johnson MJ, Bland JM, Oxberry SG, et al. . Clinically important differences in the intensity of chronic refractory breathlessness. J Pain Symptom Manage 2013; 46: 957–963. doi: 10.1016/j.jpainsymman.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 50.Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg scale, and visual analog scale. COPD 2005; 2: 105–110. doi: 10.1081/COPD-200050655 [DOI] [PubMed] [Google Scholar]
- 51.Eason AD, Parris BA. Clinical applications of self-hypnosis: a systematic review and meta-analysis of randomized controlled trials. Psychol Consciousness Theory Res Practice 2019; 6: 262–278. doi 10.1037/cns0000173 [DOI] [Google Scholar]
- 52.Similowski T, Serresse L. Lessons from negative dyspnoea studies: arguments for the multidimensional evaluation of multidirectional therapeutic approaches. Eur Respir J 2019; 53: 1802471. doi: 10.1183/13993003.02471-2018 [DOI] [PubMed] [Google Scholar]
- 53.Anlló H, Herer B, Delignières A, et al. . Hypnosis for the management of COPD-related anxiety and dyspnoea in pulmonary rehabilitation: rationale and design for a cluster-randomised, active-control trial (HYPNOBPCO_2). ERJ Open Res 2021; 8: 00565-2021. doi: 10.1183/23120541.00565-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anlló H, Larue F, Herer B. Anxiety and depression in chronic obstructive pulmonary disease: perspectives on the use of hypnosis. Front Psychol 2022; 13: 913406. doi: 10.3389/fpsyg.2022.913406 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00485-2024.SUPPLEMENT (1.4MB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00485-2024.Shareable (747.8KB, pdf)
Data Availability Statement
Data will be shared with researchers upon reasonable request.