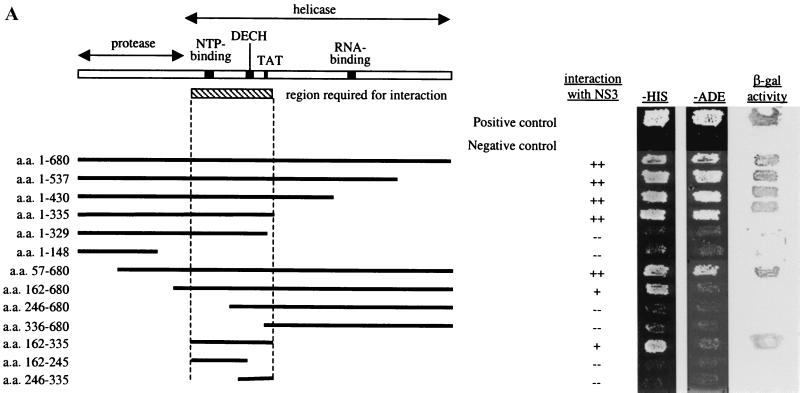
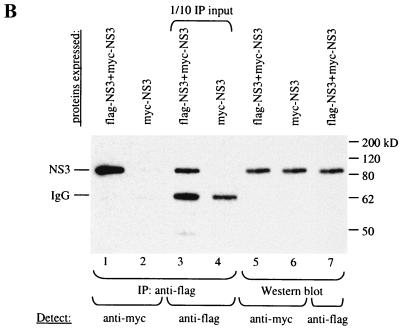
FIG. 1.
(A) A minimal domain of NS3 required for interaction was defined using the yeast two-hybrid assay. N- and C-terminal truncations in NS3 were made, and the fragments were cloned into pAS2–1. These were transformed into PJ69–2A, and transformants were mated with Y187 carrying pACT-NS3. The resulting diploids were patched onto −Trp −Leu plates, replica plated onto −His and −Ade plates, and also lifted onto a nylon membrane for the β-Gal assay. A fragment encompassing aa 182 to 335 near the N terminus of the helicase domain, which includes conserved residues important for NTP-binding, NTPase, and helicase activities, was defined to be the minimal region required for interaction with NS3. Positive interactions were indicated by growth on −His and −Ade plates and by the presence of β-Gal activity. (B) IP between Flag-NS3 and myc-tagged NS3 proteins. COS cells were transfected with Flag-NS3 and myc-NS3 or with myc-NS3 alone. Total protein (100 μg) from transfected cells was used for IP using an anti-Flag agarose gel. Lane 1, myc-NS3 was detected in the complex precipitated with an anti-Flag gel, demonstrating interaction between myc-NS3 and Flag-NS3. Lane 2, myc-NS3 was not precipitated by the anti-Flag gel in the absence of Flag-NS3, showing the specificity of the anti-Flag gel used for IP. Lanes 3 and 4, 1/10 of the input IP proteins was probed with anti-Flag antibody to show that Flag-NS3 was specifically precipitated by the anti-Flag gel. Lanes 5 through 7, Western blot of total cell lysate (20 μg loaded) to detect the expression of each of the tagged NS3 proteins in transfected cells. IgG, immunoglobulin G.