Abstract
We describe clinical characteristics and deep immunophenotypes in two patients with myelin-oligodendrocyte-glycoprotein (MOG)-antibody-associated-disease after COVID-19. The para-COVID case was a 74-year-old man who developed optic neuritis two days after COVID-19. Immunological assays revealed reduced absolute CD8+ T- and B-cell counts with increased frequency of NK cells. Post-COVID case was a 63-year-old man with optic neuritis six months after COVID-19, a frequency of CD8+ T-cells was elevated with a relatively low fraction of naïve and a high fraction of effector memory CD8+ T-cells. There was increased frequency of CD8+CD38+HLA-DR+ T-cells in the para-COVID case; interestingly, CD4+CD38+HLA-DR+ T cell frequency was increased in the post-COVID case. Both had increased SARS-CoV-2-specific and MOG-specific T-cell responses.
Keywords: myelin oligodendrocyte glycoprotein antibody-associated disease, MOGAD, SAR-CoV-2, COVID-19, optic neuritis
1. Introduction
There is growing evidence that coronavirus disease 2019 (COVID-19) or severe acute respiratory syndrome coronavirus 2 (SAR-CoV-2) infection leads to an aberrant immune response. This immune dysregulation can lead to several immune-mediated diseases, such as Guillain-Barre syndrome (Caress et al., 2020), vasculitis(McGonagle et al., 2021), inflammatory myopathies (Saud et al., 2021), and inflammatory arthropathies (Gasparotto et al., 2021). There was a limited number of case reports of myelin oligodendrocyte glycoprotein (MOG) antibody-associated-disease (MOGAD) in the setting of para-SAR-CoV-2 infection (Sawalha et al., 2020, Zhou et al., 2020). However, whether MOGAD is the immunologic sequela of COVID-19 or only a coincidence has yet to be discovered. Herein, we analyzed two cases of para- (neurological onset during active COVID-19) and post-SAR-CoV-2 (neurological onset after COVID-19 recovery) infection MOGAD with immunophenotyping and antigen-specific assays to investigate the immunologic relationship between COVID-19 and MOGAD.
2. Material and Methods
2.1. Clinical labs
Patient data were obtained through medical records at the University Hospital, Michigan Medicine, Ann Arbor, MI. Routine blood and CSF labs were done at UM hospital clinical lab. Serum aquaporin4-IgG and MOG-IgG were done at Mayo clinic using cell-based assays. Other immune assays (described below) were done in the UM Autoimmunity Center of Excellence lab.
2.2. Immunophenotyping
Blood samples were obtained after informed consent and were before steroid treatment. Immunophenotyping was done using flow cytometry. Frozen peripheral blood mononuclear cells (PBMCs) were thawed, stained with fluorochrome-conjugated antibodies, and analyzed on a BD Canto II flow cytometer and FlowJo™ version 10 (BD Life Sciences, Ashland, OR). Briefly, PBMCs were stained with antibodies against CD3, CD4, CD8, CD14, CD19, CD56, and HLA-DR to define cell lineage composition. After gating out monocyte (CD14+/FSC-Ahi), CD4 T-cells were defined as CD3+/CD56−/CD4+/CD8−; CD8 T-cells CD3+/CD56−/CD4−CD8+; natural killer T (NKT)-cells CD3+CD56+; natural killer (NK)-cells CD3−CD56+; B-cells CD3−CD56−CD19+, and Dendritic cells Lin−/HLA-DR+. CD3+ Memory T-cells were defined as CD45RA− and/or CD45RO+. Based on the expression of the lymph node-homing CC-chemokine receptor 7 (CCR7), memory T-cells were further defined as central memory T-cells (Tcm; CD45RA− or CD45RO+ and CCR7+) and effector memory T-cells (Tem; CD45RA− or CD45RO+ and CCR7+). Non-memory T-cells were defined as CD45RA+ and/or CD45RO−. Within non-memory T-cells, CCR7+ populations were defined as Naïve T-cells (Tn).
2.3. Simoa-based plasma quantification of NFL and GFAP
Frozen plasma samples from −80°C were thawed overnight at 4°C. The thawed plasma samples were spun at 12000g for 1 min before being aliquoted and stored at −80 °C before use. At the time of assay, the plasma samples were thawed at room temperature, neurofilament light chain (NFL) and glial fibrillary acidic protein (GFAP) were quantified using Quanterix’s Simoa© Neuro 2-Plex B Advantage kit according to manufacturer’s protocol (Quanterix Inc, Billerica MA. USA).
2.4. Antigen-specific T Cell Activation Assay
Frozen PBMCs derived from the non-COVID neuromyelitis optica (NMO) patient (NMO control), the patients with para-COVID-19 MOGAD, and with post-COVID-19 MOGAD were thawed and cultured for 24 hours with solvent control DMSO, or SARS-CoV-2 CD4 epitope mega peptide pool (1 ug/ml) and SARS-CoV-2 CD8 epitope mega peptide pool as provided by Sette’s group (Grifoni et al., 2020) (1 ug/ml), as well as overlapping peptide pool derived from human MOG protein. The resulting cell populations were harvested at the end of the culture and stained with fluorochrome-conjugated antibodies against CD3, CD4, CD8, CD134, CD137, CD25, CD69, and 7AAD. CD134+CD25+CD4+ T-cells represent activated antigen-specific CD4 T cells, and CD137+CD69+CD8+ T-cells represent activated antigen-specific CD8 T-cells.
3. Results
Clinical course
Case 1: Para-SAR-CoV-2 infection MOG IgG-associated optic neuritis
A 74-year-old Caucasian man presented with acute left-eye vision loss, which developed two days after mild COVID-19 symptoms. Left eye visual acuity (VA) was 20/60. A left afferent pupillary defect was noted. The rest of the neurologic examination was normal. Serum aquaporin-4 IgG was negative, and MOG IgG was positive (1:100). Cerebrospinal fluid (CSF) examination showed no pleocytosis, protein 53 mg/dl, and no oligoclonal band. Nasopharyngeal swab PCR was positive for SARS-CoV-2. Brain and orbit magnetic resonance imaging (MRI) (Fig. 1A and 1B) showed abnormal enhancement involving the intra-orbital segments of the bilateral optic nerve with mild fusiform enlargement in the left optic nerve. He received intravenous methylprednisolone 1g/day for three days. At the 6-week follow-up visit, the patient had a complete recovery. Without long-term immunotherapy, the patient remained stable at 1-year follow-up.
Figure 1. MRI images.
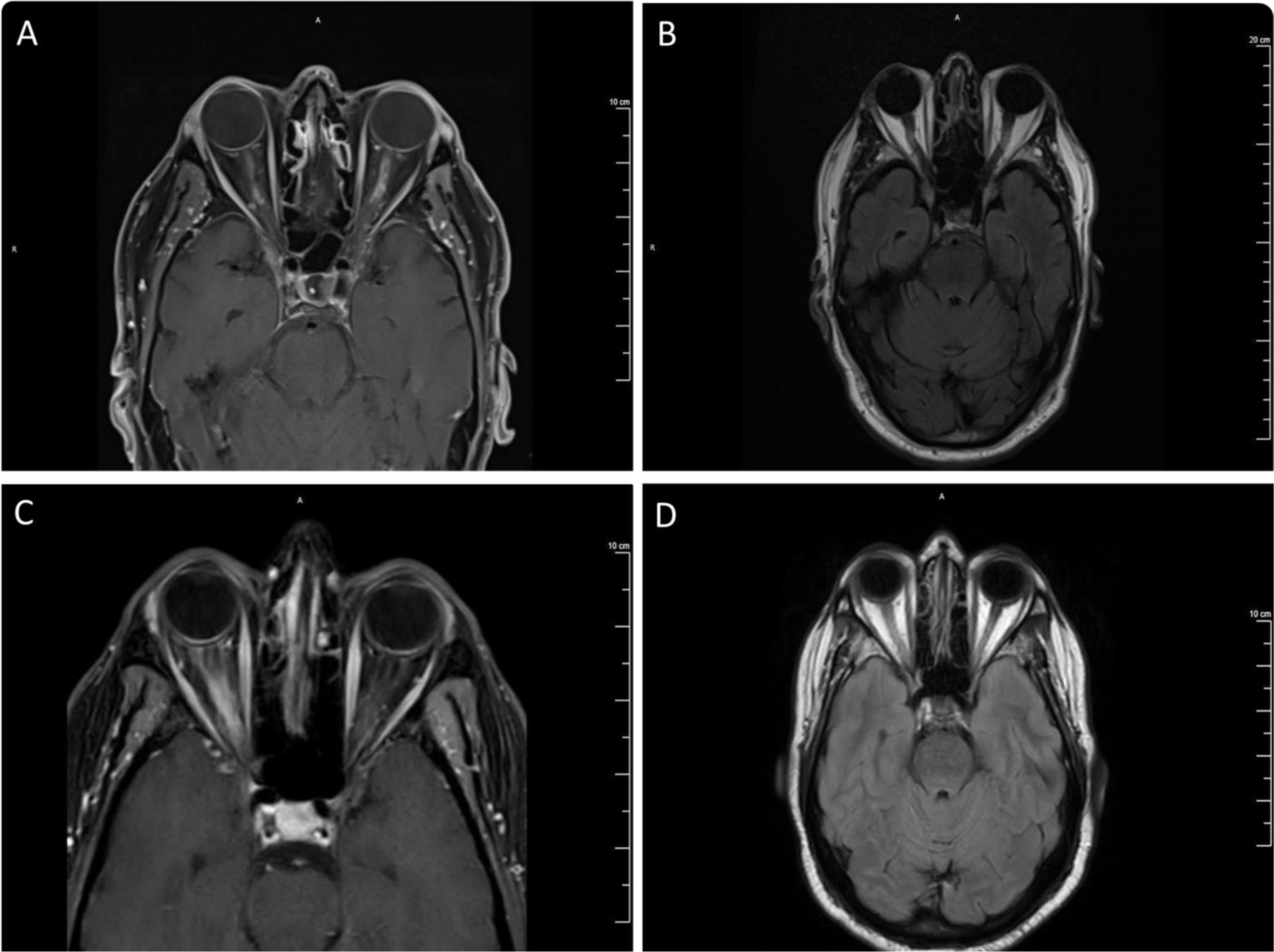
Case 1: Patchy and irregular enhancement involving the intraorbital segments of the bilateral optic nerve, left greater than right (A), with mild fusiform enlargement associated with T2 hyperintensity in the left optic nerve with preservation of surrounding subarachnoid space (B). Case 2: Abnormal enhancement (C) and T2 hyperintensity (D) in the right optic nerve extending from posterior intraorbital segment to canalicular segment with associated surrounding perineural enhancement.
Case 2: Post-SAR-CoV-2 infection MOG IgG-associated optic neuritis
A 63-year-old African American man presented with acute worsening right eye vision loss. He had poorly controlled type 2 diabetes with bilateral non-proliferative diabetic retinopathy and a history of central retinal artery occlusion of the left eye. Six months prior, he had mild COVID-19 pneumonia. His VA was finger count at 5 feet on the right eye and 20/200 on the left eye. The right pupil was poorly reactive to light. A neurological examination did not reveal any focal neurological deficit. Optical coherent tomography and fluorescein angiography were not correlated with the severity of the visual loss. Brain and orbit MRI (Fig. 1C and 1D) showed abnormal enhancement in the right optic nerve with perineural enhancement. Serum aquaporin-4 IgG was negative. Serum MOG IgG was positive (1:1000). CSF showed no pleocytosis, protein 107 mg/dl, and no oligoclonal band. He received a 3-day course of intravenous methylprednisolone 1g/day. One week after systemic corticosteroid therapy, his VA was 20/150 on the right eye and 20/200 on the left eye. Because of pre-existing poor vision in the left eye, the patient was started on oral mycophenolate mofetil along with oral prednisone taper after completing intravenous steroid. At the 6-month follow-up, his VA recovered to baseline.
Immunophenotyping of flow cytometry assay
Compared with age-matched healthy control, immunophenotyping of case 1 showed some noticeable features, such as lower frequency (3.73%) and absolute numbers (0.08K/μl) of CD8+ T-cells, as well as reduced absolute numbers of B-cells (0.07K/μl), along with the very high frequency of NK-cells (20.68%) and higher frequency of HLA-DR+ CD3+CD56+ cells (14.1%) (Table 1). The FoxP3+ CD4+ T-cells showed a relatively high frequency of proliferation but a lower frequency of interleukin (IL)-10 expression (Table 1). A normal frequency of Tn, Tcm, Tem, and Temra (defined in the method section) was found.
Table 1. PBMC Immune Profiling.
PBMCs derived from three patients including a control from age and sex matched untreated neuromyelitis optica patient without COVID, MOG optic neuritis with COVID patient (para-COVID ON), MOG optic neuritis 6 month post covid patients (post-COVID ON), and age and sex matched healthy controls (n=5) were stained with fluochrome-conjugated antibodies against different cell lineage markers and analyzed using flow cytometry. With limitation of our flow panel, natural killer T (NKT) cells were simply estimated as CD3+CD56+. Details are described in method section. The values that are larger than the mean + 2xSD of corresponding healthy controls are in red. The values that are less than the mean-2xSD of corresponding healthy controls are in blue.
| Patients: | Parameters | HC (Mean±SD) | Non-Covid NMO | ON with Covid | ON post-Covid |
|---|---|---|---|---|---|
| PBMC Cell Lineages | |||||
| % of PBMC | Monocyte (%) | 25.6±11.3 | 9.58 | 14.7 | 7.92 |
| CD4+ T (%) | 31.7±13.0 | 55.68 | 42.13 | 26.57 | |
| CD8+ T (%) | 9.7±4.7 | 8.48 | 3.73 | 27.26 | |
| CD4/CD8 | 4.5±4.1 | 6.57 | 11.29 | 0.97 | |
| CD3+CD56+ (%) | 1.9±1.5 | 0.78 | 0.93 | 4.1 | |
| NK (%) | 7.1±2.5 | 7.9 | 20.68 | 3.08 | |
| B cells (%) | 4.5±1.7 | 5.46 | 3.39 | 5.99 | |
| Dendritic (%) | 2.5±1.5 | 0.99 | 1.32 | 1.15 | |
| Absolute # * | Monocyte (K/uL) | rangea (0.1–1.1) | 0.2 | 0.31 | 0.21 |
| CD4+ T (K/uL) | rangeb (0.46–1.72) | 1.17 | 0.88 | 0.72 | |
| CDS+ T (K/uL) | rangeb (0.135–0.852) | 0.18 | 0.08 | 0.74 | |
| CD3.CD56+ (K/uL) | NA | 0.02 | 0.02 | 0.11 | |
| NK (K/uL) | rangeb (0.082–0.594) | 0.17 | 0.43 | 0.08 | |
| B cells (K/uL) | rangeb (0.092–0.515) | 0.11 | 0.07 | 0.16 | |
| Lymphocyte (K/uL) | rangea (1.2–4.0) | 1.65 | 1.48 | 1.81 | |
| Dendritic (K/uL) | NA | 0.02 | 0.03 | 0.03 | |
| HLA-DR+% | Monocyte (%) | 99.5±0.5 | 97.2 | 98.9 | 96.9 |
| CD4+ T (%) | 9.7±4.7 | 1.47 | 5.37 | 6.28 | |
| CD8+ T (%) | 6.4±1.9 | 3.58 | 10.1 | 6.63 | |
| CD3+CD56+ (%) | 4.1±1.9 | 2.96 | 14.1 | 7.87 | |
| NK (%) | 5.7±3.3 | 1.62 | 1.57 | 9.85 | |
| B cells (%) | 97.7±1.8 | 99.7 | 98.2 | 99.3 | |
| Dendritic (%) | 100 | 100 | 100 | 100 | |
| T memory Subsets | |||||
| CD4+ T | Tn (%) | 41.8±20.8 | 68.9 | 52.9 | 14.5 |
| Tcm (%) | 24.1±10.3 | 20 | 29.4 | 30.6 | |
| Tem (%) | 29.1±15.7 | 9.87 | 16.2 | 44.3 | |
| Temra (%) | 5.0±6.6 | 1.24 | 1.45 | 10.6 | |
| CD8+ T | Tn (%) | 29.3±9.0 | 27.4 | 13.2 | 5.96 |
| Tcm (%) | 10.8±8.0 | 9.93 | 14.7 | 5.74 | |
| Tem (%) | 48.1±8.2 | 53.8 | 54.3 | 40.1 | |
| Temra (%) | 11.8±10.2 | 8.93 | 17.9 | 48.2 | |
| T helper Subsets | |||||
| T Helpers | Th1–17 (% of CD4+ T) | 16.1±7.6 | 4.58 | 8.89 | 32.99 |
| Th1 (% of CD4+ T) | 20.1±3.8 | 11.91 | 25.6 | 19.21 | |
| Th17 (% of CD4+ T) | 16.7±6.5 | 11.26 | 13.13 | 27.03 | |
| Th2 (% of CD4+ T) | 46.2±11.1 | 69.74 | 51.26 | 19.62 | |
| Tfh Subsets | Tfh1–17 (% of Tfh) | 25.2±11.5 | 9.43 | 16.91 | 24.5 |
| Tfh1 (% of Tfh) | 19.8±5.5 | 15.29 | 27.7 | 19.76 | |
| Tfh17 (% of Tfh) | 22.3±16.1 | 33.71 | 16.3 | 37.9 | |
| Tfh2 (% of Tfh) | 32.0±17.8 | 39.09 | 37.82 | 17.11 | |
| Treg | |||||
| CD25+ FoxP3+ (% of CD4+ T | 1.5±1.3 | 3.01 | 4.01 | 7.31 | |
| IL-10+.% of Treg (%) | NA | 12.8 | 3.7 | 4.2 | |
| Ki 61+% of Treg (%) | NA | 6.58 | 15.1 | 13.6 | |
Absolute # of normal control are presented as normal ranges:
given by clinical lab of University of Michigan or
from reference (Apoil et al, 2017)
Immunophenotyping of case 2 showed a significantly higher frequency of CD8+ T cells (27.26%), of which only very low frequency (5.96%) were naïve cells with very high frequency (48.2%) of terminally differentiated effector cells (Temra). This patient had a higher frequency of pro-inflammatory CXCR3+CCR6+ and/or CXCR3+CD161+ Th1–17 cells (32.99%) along with a lower frequency of anti-inflammatory Th2 cells (19.62%, Table 1). A relatively high frequency of regulatory T-cells (Treg) (7.31%) was also found (Table 1). The FoxP3+ CD4+ T-cells showed higher proliferation frequency (ki67+%=13.6) compared with FoxP3− counterpart (Ki67+%=4.0% data not shown). However, its IL-10+ frequency (4.2%) is much lower than the NMO control (12.8%).
Case 1 showed CD38+HLA-DR+ population was enriched in CD4− T- cells but not in CD4+ T-cells (Fig. 2A). These enriched CD8+CD38+HLA-DR+ activated T-cells were found to be predominantly CD45RO+CCR7− (60.7%) therefore belonging to the effector memory T-cell (Tem) subset. Within this, a CD38hiHLA-DRhi subgroup was found, which contains a similar but somewhat higher frequency of Tem (64.2%, data not shown). Case 2 showed CD38+HLA-DR+ T-cells were enriched in CD4+ T-cells (Fig. 2A). Most (85.8%) of the activated CD38+HLA-DR+ CD4+ T-cells belong to the effector memory T-cell (Tem) subset.
Figure 2: Active SARS-CoV-2 and MOG-specific T cell responses with neuroaxonal damage.
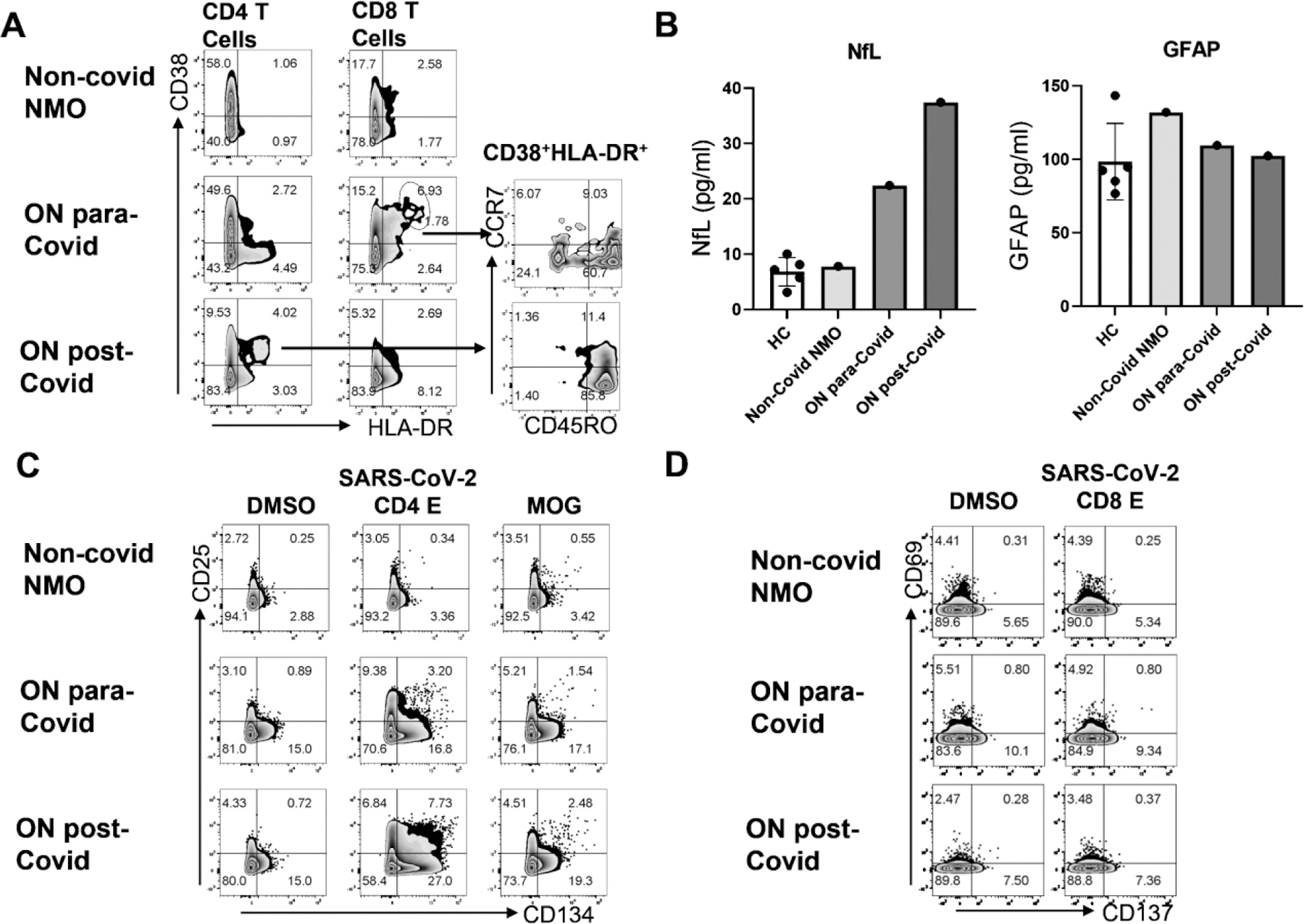
A. Activated T cells (CD38+HLA-DR+) were detectable in PBMC derived from Covid ON patients. B. Plasma neurofilament light chain level, but not GAFP level are higher in Covid ON patients compared to healthy control or non-covid neuromyelitis optica patient. C. Antigen specific CD4+ T cell activation (CD25+CD134+) in response to 24hr in vitro stimulation with solvent control DMSO, or SARS-CoV-2 CD4 epitope mega peptide pool (1ug/ml) (Grifoni et al., 2020) or human MOG overlapping peptide pool (1ug/ml). D. Antigen specific CD8+T cell activation (CD69+CD137+) in response to 24hr in vitro stimulation with solvent control DMSO, or SARS-CoV-2 CD8 epitope mega peptide pool (Grifoni et al., 2020) (1ug/ml).
NFL and GFAP assay
Since NFL levels were found to have prognostic value for optic neuritis, plasma NFL (pNFL) and GFAP were quantitatively measured (Fig. 2B) using Simoa technology developed by Quanterix (Hendricks et al., 2019). Compared to healthy control, pNFL, but not GFAP, was abnormally increased: 2 fold of HC in Case 1 and 4-fold of HC in Case 2, suggesting increased neuroaxonal damage.
COVID-specific and MOG-specific T cell assay
SARS-CoV-2 and MOG-specific-T-cell response was analyzed using an in vitro antigen-specific T-cell stimulation assay. When PBMCs were stimulated with CD4 T-cell-targeted MegaPool derived from SARS-CoV2 (Grifoni et al., 2020), Case 1 showed increased frequency of CD25+CD134+ (3.2% vs. 0.34% as compared with NMO control) in CD4+CD8−T (Fig. 2C); Case 2 showed higher frequency of CD25+CD134+ (7.73%) in CD4+CD8−T-cells. In Case 1, MOG-specific activated CD25+CD134+ T-cells were found in CD4+ T cells (1.54% vs. 0.55%), which collaborated with 1:100 anti-MOG antibody titre in this patient (Fig. 2C). In Case 2, anti-MOG antibody titre was 1:1000, MOG-specific CD4 T-cell response was more prominent (2.48%) than Case 1 and the NMO control (0.56%, Fig. 2C).
When PBMCs were stimulated with CD8 T-cell-targeted MegaPool derived from SARS-CoV2, a low frequency, but SARS-CoV-2-specific activated CD69+CD137+ population (0.8% vs. 0.25%) was detected within CD4−CD8+ T-cells in Case 1 only, but not in Case 2 (Fig. 2D). Very little, if any, anti-SARS-CoV-2-specific CD4−CD8+ T-cells were detected in Case 2 (Fig. 2D).
In both cases, there were positive humoral immune responses to spike RBD and nucleocapsid antibody with commercial assay (described in Ali et al., 2021).
4. Discussion
We described two MOGAD cases temporally related to COVID-19 and both cases were in early 2020 when patients had COVID for the first time and there no COVID vaccine available. Although both experienced acute optic neuritis, their temporal relationship with COVID differed. Case 1 MOGAD symptoms started 2 days after the patient tested positive for SARS-CoV2 with mild COVID symptoms, while Case 2 started to have vision loss 6 months after COVID pneumonia.
Peripheral blood from Case 1, who was in an early phase of SARS-CoV2 infection, showed strong innate immune responses (increased NK cells) along with reduced frequency and absolute number CD8+ T-cells. Within CD8+ T-cells population, we detected a CD38hiHLA-DRhi subset, usually found in patients with SARS-CoV2 infections and other viral infections. Few SARS-CoV-2-specific CD8+ T-cells were detected in this patient.
Peripheral blood from Case 2, who had recovered from COVID showed significantly expanded CD8+ T-cells, along with normal frequency and an absolute number of CD4+ T cells. But within CD8+ T-cells, much lower, if any, SARS-CoV-2-specific T cells were detected, suggesting the passing of active anti-SARS-CoV-2 CD8 T-cell responses when a patient recovered from COVID-19. Interestingly, however, instead of CD8+ T-cells, we found a distinct CD38+HLA-DR+ population of T cells in CD4+ T-cells. CD4+CD38+HLA-DR+ representing activated T-cells were previously described in HIV or HCV-infected individuals (d’Ettorre et al., 2016). This population has not been previously studied for SARS-CoV-2 infection. It is possible that these activated CD4+CD38+HLA-DR+ cells were induced by SARS-CoV-2 infection or represented an ongoing chronic inflammation. It could also be directly involved in the pathogenesis of ON. More analysis of this population in post-COVID patients would be warranted to see if it could be a potential biomarker for monitoring auto-immune-prone individuals. CD4+CD38+ HLA-DR+ cells described in HIV- infected patients expressed elevated levels of CCR5 and CXCR4 (Meditz et al., 2011). Both chemokine receptors were found to be involved in the pathogenesis of autoimmune diseases, so it is possible that these cells were involved in pathogenesis in MOGAD (Nagafuchi et al., 2016, Szczuciński and Losy, 2007).
Correlating to their clinical history of COVID, SARS-CoV2-specific CD4+ and CD8+ T-cells were detectable, which were significantly higher than NMO control. Autoimmune CD4+ T-cells specific to MOG were also clearly detectable in both MOGAD cases. The MOG-specific CD4 T-cells may facilitate the production of anti-MOG antibodies in these individuals.
Besides the overtly active immune profile presented by case 2, highly proliferating cells were also noticeable. However, these cells produced less amount of IL-10 compared to NMO control, implying that they could be dysfunctional, which may be responsible for the inability to damp down the overly active immune system in a timely fashion.
The limitation is that our case study only contains a limited number of cases and controls. Our findings are descriptive. Future larger study will be needed to confirm the findings. Immunoglobulin and complement measurements were not routinely measured in clinical practice and most of testing was in the blood and we noted some CSF findings as the above. NFL is one of the major cytoskeletal proteins present in neuronal axons. It can be released into CSF and blood if axons are damaged. Plasma levels of NFL (pNFL) are found to be correlated with CSF levels (Novakova et al., 2017). Simoa sensitive assay allows pNFL to be used a surrogate maker for axonal damage. Compared to age-matched healthy controls, pNFL in both MOGON cases were increased. Our data was the first demonstration of neuroaxonal damage in MOGON post COVID.
Though the causality of these MOGAD cases is still unknown, the temporal course of our cases suggested that either acutely around COVID-19 infection or months after, MOGAD could occur. Recent series described nine patients with MOGAD following COVID-19 (Lambe et al., 2022), though global incidents of MOGAD since the pandemic remains unknown. Our 6-month post COVID MOGAD case could be potentially viewed as idiopathic MOGAD as COVID history may or may not be related development of MOGAD. The post-COVID MOGAD is still quite plausible since we were able to detect very strong CD4 response to SARS-CoV2 peptide pool in this patient (Fig. 2C), although his CD8 response to SARS-CoV2 was relatively weak (Fig. 2D) compared with para-COVID case. We showed that MOG-specific T-cell response was enhanced in para-infectious or post-infectious of COVID-19, which may be amplified by the anti-SARS-CoV2 response. Potential mechanisms may include enhanced T cell antigen response via molecular mimicry and bystander activation. Most patients seemed to have great response to steroids treatment and do not need long term immunosuppressant (Lambe et al., 2022). This aspect differs from multiple sclerosis as long-term treatment is needed.
5. Conclusion
Our data provide initial preliminary insight into one potential immunopathogenesis of MOGAD by the heightened antigen-specific-T-cell response with infection. However, the infectious trigger of various autoimmunity likely varies from person to person.
Highlight.
A new onset of MOG-antibody associated disease can occur as para- or post-SAR-CoV-2 infection.
There were notable cytotoxic CD8 and innate NK cell changes.
There was increased frequency of CD8+CD38+HLA-DR+ T-cells in the para-COVID case.
CD4+CD38+HLA-DR+ T cell frequency was increased in the post-COVID case.
There were enhanced MOG-specific and SARS-CoV-2-specific T-cell responses in para- and post-SAR-CoV-2 infection
Acknowledgment
We are genuinely grateful for Dr. Alessandro Sette’s group in the Center for Infectious Disease and Vaccine Research, La Jolla Institute of Immunology who kindly provided us their SARS-CoV-2 virus-specific CD4 and CD8 Epitope MegaPool used in this study. We sincerely thank our patients and family for participating in the study.
Disclosure
Y Mao-Draayer has served as a consultant and/or received grant support from: Acorda, Bayer Pharmaceutical, Chugai, Biogen Idec, EMD Serono, Sanofi-Genzyme, Genentech, Novartis, Horizon, Janssen, Questor, and Teva Neuroscience. DA Fox and Y Mao-Draayer were supported by grants from NIH NIAID Autoimmune Center of Excellence: UM1-AI110557-05 and UM1 AI144298-01. Y Mao-Draayer was also supported by PCORI, Novartis, Sanofi-Genzyme, Genentech, and Chugai. Other authors have nothing to disclose.
6. Funding
This work was supported by grants from NIH NIAID Autoimmune Center of Excellence: UM1-AI110557-05, UM1 AI144298-01, NIH NINDS R01-NS080821, PCORI, Novartis, Sanofi-Genzyme, and Chugai.
Abbreviation
- COVID-19
Coronavirus-19
- CCR7
CC motif chemokine receptor 7
- CSF
Cerebrospinal fluid
- GFAP
Glial fibrillary acidic protein
- IL
Interleukin
- MOG
Myelin oligodendrocyte glycoprotein
- MOGAD
Myelin oligodendrocyte glycoprotein antibody-associated disease
- MRI
Magnetic resonance imaging
- NFL
Neurofilament light chain
- NK cell
Natural killer cell
- NKT cell
Natural killer T-cell
- NMO
Neuromyelitis optica
- ON
Optic neuritis
- PBMC
Peripheral blood mononuclear cell
- SAR-CoV-2
Severe acute respiratory syndrome coronavirus 2
- Tcm
Central memory T-cell
- Tem
Effector memory T-cell
- Temra
Terminally differentiated effector T-cell
- Tn
Naïve T-cell
- Treg
Regulatory T-cell
- VA
Visual acuity
7. References
- Ali A, Dwyer D, Wu Q, Wang Q, Dowling CA, Fox DA, Khanna D, Poland GA, Mao-Draayer Y. Characterization of humoral response to COVID mRNA vaccines in multiple sclerosis patients on disease modifying therapies. Vaccine. 2021. Oct 1;39(41):6111–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apoil PA, Puissant-Lubrano B, Congy-Jolivet N, Peres M, Tkaczuk J, Roubinet F, & Blancher A (2017). Reference values for T, B and NK human lymphocyte subpopulations in adults. Data in brief, 12, 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caress JB, Castoro RJ, Simmons Z, Scelsa SN, Lewis RA, Ahlawat A & Narayanaswami P 2020. COVID-19-associated Guillain-Barré syndrome: The early pandemic experience. Muscle Nerve, 62, 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’ettorre G, Ceccarelli G, Serafino S, Giustini N, Cavallari EN, Bianchi L, Pavone P, Bellelli V, Turriziani O, Antonelli G, Stroffolini T & Vullo V 2016. Dominant enrichment of phenotypically activated CD38(+) HLA-DR(+) CD8(+) T cells, rather than CD38(+) HLA-DR(+) CD4(+) T cells, in HIV/HCV coinfected patients on antiretroviral therapy. J Med Virol, 88, 1347–56. [DOI] [PubMed] [Google Scholar]
- Gasparotto M, Framba V, Piovella C, Doria A & Iaccarino L 2021. Post-COVID-19 arthritis: a case report and literature review. Clin Rheumatol, 40, 3357–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, De Silva AM, Frazier A, Carlin AF, Greenbaum JA, Peters B, Krammer F, Smith DM, Crotty S & Sette A 2020. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell, 181, 1489–1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks R, Baker D, Brumm J, Davancaze T, Harp C, Herman A, Büdingen HV, Townsend M & Fischer SK 2019. Establishment of neurofilament light chain Simoa assay in cerebrospinal fluid and blood. Bioanalysis, 11, 1405–1418. [DOI] [PubMed] [Google Scholar]
- Lambe J, Mcginley MP, Moss BP, Mao-Draayer Y, Kassa R, Ciotti JR, Mariotto S & Kunchok A 2022. Myelin oligodendrocyte glycoprotein-IgG associated disorders (MOGAD) following SARS-CoV-2 infection: A case series. J Neuroimmunol, 370, 577933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgonagle D, Bridgewood C, Ramanan AV, Meaney JFM & Watad A 2021. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol, 3, e224–e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meditz AL, Haas MK, Folkvord JM, Melander K, Young R, Mccarter M, Mawhinney S, Campbell TB, Lie Y, Coakley E, Levy DN & Connick E 2011. HLA-DR+ CD38+ CD4+ T lymphocytes have elevated CCR5 expression and produce the majority of R5-tropic HIV-1 RNA in vivo. J Virol, 85, 10189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi Y, Shoda H, Sumitomo S, Nakachi S, Kato R, Tsuchida Y, Tsuchiya H, Sakurai K, Hanata N, Tateishi S, Kanda H, Ishigaki K, Okada Y, Suzuki A, Kochi Y, Fujio K & Yamamoto K 2016. Immunophenotyping of rheumatoid arthritis reveals a linkage between HLA-DRB1 genotype, CXCR4 expression on memory CD4(+) T cells, and disease activity. Sci Rep, 6, 29338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakova L, Zetterberg H, Sundström P, Axelsson M, Khademi M, Gunnarsson M, Malmeström C, Svenningsson A, Olsson T, Piehl F, Blennow K & Lycke J 2017. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology, 89, 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saud A, Naveen R, Aggarwal R & Gupta L 2021. COVID-19 and Myositis: What We Know So Far. Curr Rheumatol Rep, 23, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawalha K, Adeodokun S & Kamoga GR 2020. COVID-19-Induced Acute Bilateral Optic Neuritis. J Investig Med High Impact Case Rep, 8, 2324709620976018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczuciński A & Losy J 2007. Chemokines and chemokine receptors in multiple sclerosis. Potential targets for new therapies. Acta Neurol Scand, 115, 137–46. [DOI] [PubMed] [Google Scholar]
- Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ & Patel VR 2020. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Optic Neuritis and Myelitis in COVID-19. J Neuroophthalmol, 40, 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]


