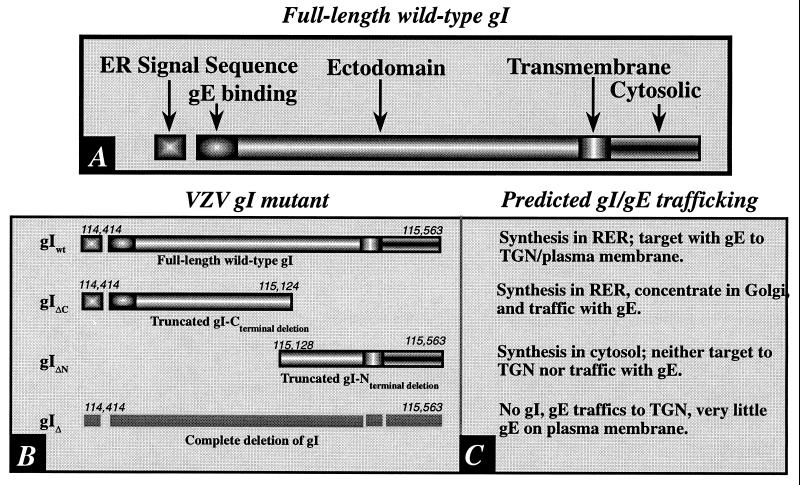
FIG. 1.
(A) The domains of gI are shown diagrammatically. Note that the signal sequence, which is presumably responsible for the biosynthesis of gI in the RER, is at the N-terminal end of the molecule. Because signal peptides are cleaved cotranslationally, this domain is not found in the completed integral membrane protein. (B) The domains of the tested mutant forms of gI are compared to those of the full-length intact gI. The numbers refer to base pairs in the VZV genome at which the sequence encoding the indicated domain begins or ends. (C) The predicted patterns of gI trafficking in cells infected with the corresponding virions diagrammed in panel B. These predictions were tested in immunocytochemical experiments. (i) gIwt. The trafficking of gI in cells infected with intact VZV has previously been described (30) and is thus the expected pattern in cells infected with intact virions. Since gI and gE form a complex in the RER, they would be expected to traffic together during post-RER stages of intracellular transport. (ii) gIΔC. Despite the deletion of its C-terminal domain, the gIΔC mutant protein would still be expected to be synthesized in the RER because it contains a signal sequence; however, since the transmembrane domain is lacking, the gIΔC mutant should be completely translocated to the lumen of the RER and lack a membranous anchor. Although gIΔC is thus analogous to a secreted protein, the complex formed with gE at its N-terminal would be expected to cause the gIΔC mutant to be transported along with its normal gE partner. (iii) gIΔN. The deletion of the N-terminal domain of gI (gIΔN) would be anticipated to prevent its biosynthesis in the RER because of the lack of a signal sequence. The deletion of the N-terminal domain of gI would also be expected to prevent interactions of gIΔN with gE. The elimination of these interactions would not be expected to interfere with the targeting of gE to the TGN because gE has its own TGN targeting sequence and patch (37). (iv) gI130. The total deletion of gI would, of course, be expected to eliminate gI immunoreactivity. Because of the endocytosis signal in the sequence of gE (22), relatively little gE would be expected to be retained on the plasma membrane unless it were induced to remain there because it is complexed with gI. gI is not retrieved to the TGN when it traffics to the plasma membrane in transfected cells that express only gI (1, 30; but see also reference 21). The retention of gE in the plasma membrane might thus occur in cells infected with virions carrying gIwt but not in those carrying gIΔN or gIΔ. Because the gIΔC protein is not membrane anchored, it also would not be expected to interfere with the endocytosis of the gE to which it is bound.