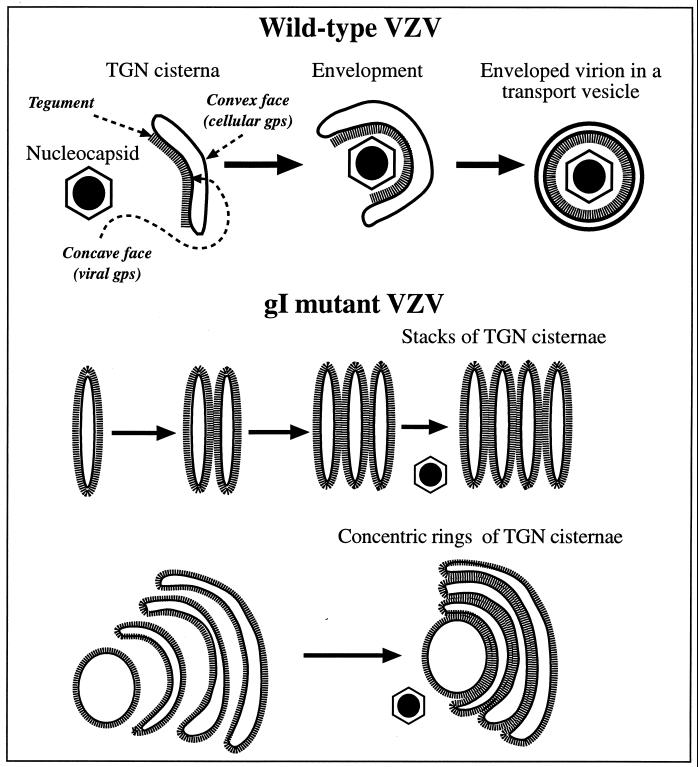
FIG. 13.
A heuristic model depicting the role of TGN cisternae in viral envelopment in cells infected with intact (Ellen) VZV and how the envelopment process becomes defective in cells infected with gIΔ, gIΔC, or gIΔN mutant virions. When cells are infected with intact (Ellen) VZV (upper panel), nucleocapsids that are free in the cytosol become associated with specialized wrapping cisternae in the TGN. These cisternae are curvilinear, with distinct concave and convex faces. Tegument adheres to the concave face of the curving cisternae, and as the arms of the wrapping cisternae approach one another and ultimately fuse, tegument and the nucleocapsid are enclosed within. The membrane of the concave face of the wrapping cisternae, which is proposed to be rich in viral glycoproteins, becomes the viral envelope. The membrane of the convex face, which is rich in cellular proteins, such as Man 6-P receptors, delimits a transport vesicle that encloses the newly enveloped virion. When gI is deficient (lower panels), viral proteins are no longer segregated to one face of the TGN cisternae. As a result the tegument, which is presumed to bind to the cytosolic domains of the viral glycoproteins, is not confined to a single surface of the TGN cisternae. Because tegument is not restricted to the concave face of curving cisternae, adjacent tegument-coated cisternae fuse with one another to give rise to stacks and concentric rings of adherent sacs. The defect in the TGN interferes with viral envelopment and with the post-Golgi transport of VZV.