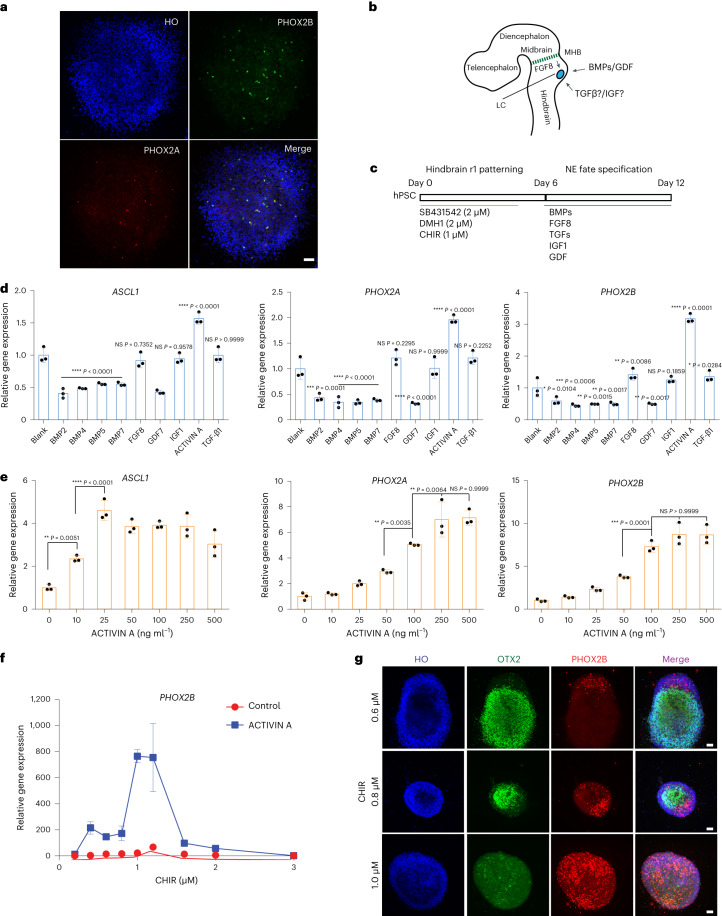
Fig. 2. Specification of NE progenitors.
a, Immunostaining for NE neural progenitor markers PHOX2A and PHOX2B at day 6 in cells treated with 1.0 µM CHIR99021 (CHIR). HO, Hoechst. Scale bar, 50 µm. b, Schematic representation of the potential morphogens that may affect NE progenitor fate specification. c, Experimental design to identify factors that positively affect NE progenitor specification. d, qPCR of NE progenitor markers ASCL1, PHOX2A and PHOX2B under the treatment of BMPs, FGF8, GDF7, IGF1, ACTIVIN A and TGFβ1. Data are shown as mean ± s.d. n = 3 biologically independent samples for each condition. The significance (versus ‘Blank’ condition) was assessed by one-way ANOVA (Dunnett’s multiple comparisons test). *P < 0.05, **P < 0.01,***P < 0.001 and ****P < 0.0001. NS, not significant. e, qPCR of NE progenitor markers ASCL1, PHOX2A and PHOX2B under a series of ACTIVIN A concentrations. Data are shown as mean ± s.d. n = 3 biologically independent samples for each condition. The significance was assessed by one-way ANOVA (Dunnett’s multiple comparisons test). *P < 0.05, **P < 0.01 and ***P < 0.001. NS, not significant. f, Relative PHOX2B expression in the presence or absence of ACTIVIN A (2nd week), whereas the cells were treated with a series of CHIR99021 concentrations at the first week. Data are shown as mean ± s.d. n = 3 biologically independent samples for each condition. g, Immunostaining for the regional marker OTX2 and NE progenitor marker PHOX2B at day 12 when cells were treated with 125 ng ml−1 ACTIVIN A. Scale bar, 50 µm.