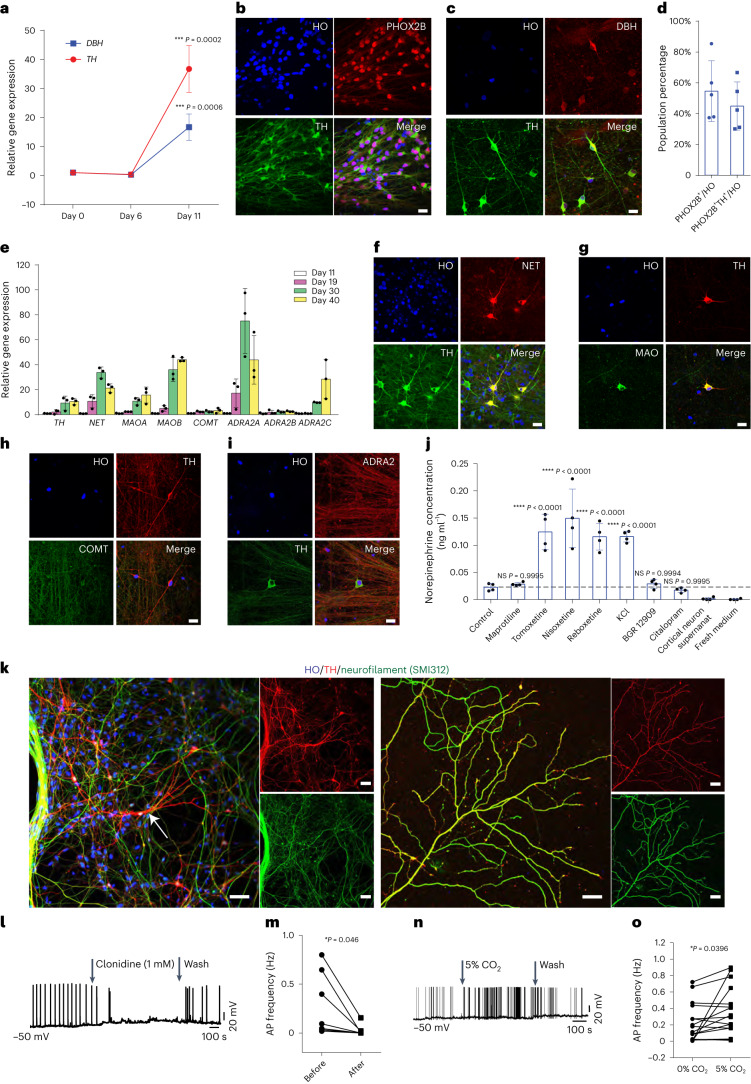
Fig. 4. Maturation of NE neurons.
a, qPCR of NE neuronal marker genes TH and DBH during differenitation. Data are shown as mean ± s.d. n = 3 biologically independent samples for each condition. The significance (versus day 0) was assessed by one-way ANOVA (Dunnett’s multiple comparisons test) for each gene. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. NS, not significant. b,c, Immunostaining of NE neuronal markers PHOX2B, TH and DBH at day 18 (b) and day 30 (c). HO, Hoechst. Scale bar, 20 µm. d, Quantification of PHOX2B and PHOX2B/TH+ cells in culture. Data are shown as mean ± s.d. n = 5 biologically independent samples for each condition. e, qPCR analysis of gene expression after neuronal differentiation at days 11, 19, 30 and 40. Data are shown as mean ± s.d. n = 3 biologically independent samples for each condition. f–i, Immunostaining of NE markers NET, MAO, COMT and ADRA2 in H9-dervied NE neurons at day 30. Scale bar, 20 µm. j, Supernatant NE content at week 4 under the treatment of NRIs or KCl. Data are shown as mean ± s.d. n = 4 biologically independent samples for each condition. Significance (versus control group) was assessed by one-way ANOVA (Dunnett’s multiple comparisons test). *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. k, Immunostaining of neurofilament in NE neuron cell body, dendrites and axons. The white arrow points to the NE cell body, which is not stained by neurofilament (SM312). Scale bar, 50 µm. l, Representative trace of spontaneous firing before, at and after clonidine (1 mM) treatment. m, Quantification of the firing rate change in l. Data are shown as symbols and lines in the ‘before–after’ pattern. n = 6 neurons. n, Representative trace of spontaneous firing before, at and after perfusion with 5% CO2. o, Quantification of the firing rate change in n. Data are shown as symbols and lines in the ‘before–after’ pattern. n = 16 neurons. Significance was assessed by paired t-test (two-tailed) in m,o.