FIG. 6.
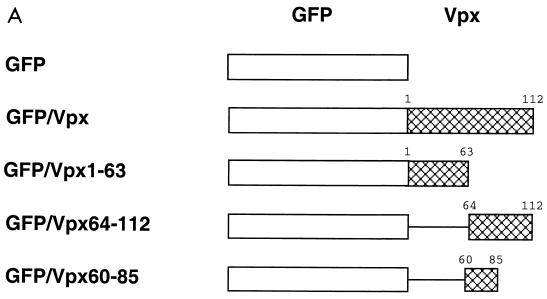
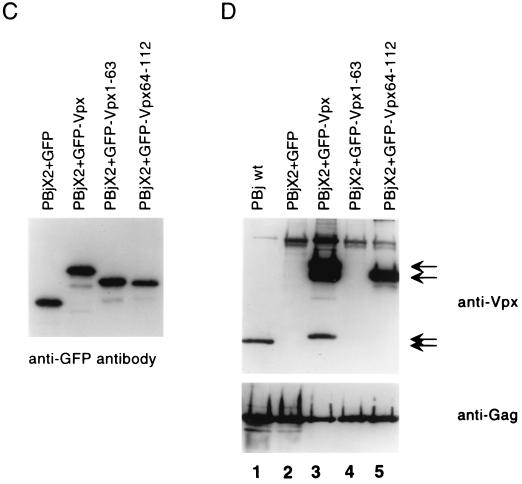
Construction, subcellular localization, expression and packaging of GFP-Vpx fusion proteins. (A) Schematic representation of GFP-Vpx fusion proteins. (B) Subcellular localization of GFP-Vpx fusion proteins in HeLa cells (see Results for details). Subpanels: a, the nuclear membrane was visualized by indirect immunofluorescence using a nucleoporin p62 specific polyclonal antibody, followed by a Texas red-conjugated goat anti-rabbit secondary antibody (red), and DAPI was used for nuclear staining (blue); b, the GFP signal was used to localize the GFP-Vpx fusion proteins; c, superimposition of images shown in subpanels a and b for each row. (C) Expression of GFP-Vpx fusion proteins. 293T cells were transfected with various GFP-Vpx expression plasmids. Lysates of transfected cells were prepared 48 h after transfection and analyzed for fusion protein content by Western blot analysis using an anti-GFP antibody. (D) Packaging of GFP-Vpx fusion proteins. Expression plasmids were cotransfected with the PBj1.9 Vpx− proviral clone X2, and virus particles were concentrated from culture supernatants by ultracentrifugation through a 20% sucrose cushion, solubilized in gel loading buffer, and analyzed for protein content by Western blot analysis using Vpx (top)- and Gag (bottom)-specific monoclonal antibodies. Arrows indicate proteins with Vpx reactivity. Lane 1, wild-type Vpx protein (18 kDa); lane 2, negative (GFP) control; lane 3, full-length GFP-Vpx fusion protein (46 kDa) as well as two smaller proteins (38 kDa and 20 kDa, respectively) likely representing protease cleavage products; lane 4, lack of GFP-Vpx1-63 packaging; lane 5, GFP-Vpx64-112 fusion protein (38 kDa).