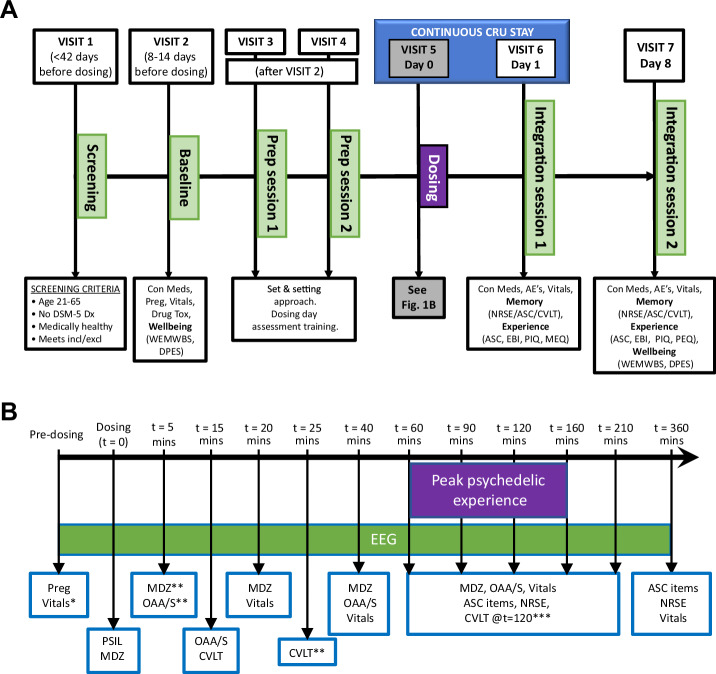
Fig. 1. Study schema.
A Experimental schedule. AE adverse event, ASC altered states of consciousness questionnaire, CEQ challenging experiences questionnaire, Con Med concomitant meds, CVLT California verbal learning task, DPES dispositional positive emotion scales, Drug tox urine drug screen, EBI emotional breakthrough inventory, MEQ mystical experiences questionnaire, NRSE narrative report of subjective experience, PEQ persisting effects questionnaire, PIQ psychological insight questionnaire, Preg pregnancy test, Vitals = blood pressure, heart rate, pO2; WEMWBS Warwick Edinburgh mental well-being scale. B Dosing day schedule. MDZ midazolam, OAA/S Observer’s assessment of arousal and sedation, Preg pregnancy test, PSIL psilocybin. *Vitals = blood pressure, heart rate, pO2. **Participants 3–8 only. ***Participants 5–8 only.