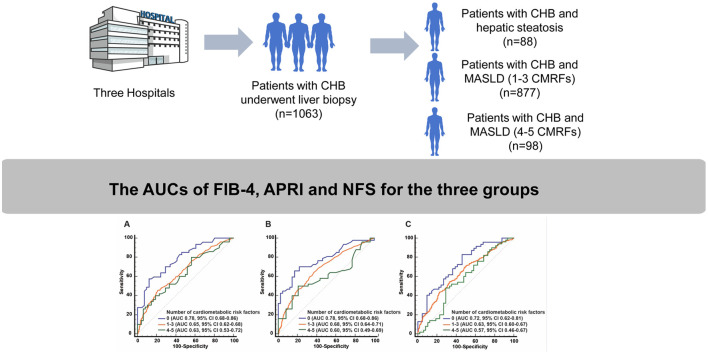
Graphical abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD), is increasingly prevalent in Asia,1 an area also endemic for chronic hepatitis B (CHB). Our previous study showed that the coexistence of CHB and hepatic steatosis (HS) has reached 36.5% in Asia,2 with a similar figure observed globally.3 This coexistence may exacerbate the progression of hepatic fibrosis. Non-invasive tests (NITs) like the fibrosis-4 index (FIB-4),4 aspartate aminotransferase-to-platelet ratio index (APRI),5 and the NAFLD fibrosis score (NFS)6 are crucial for diagnosing hepatic fibrosis. While previous studies have revealed that these NITs have suboptimal accuracy for diagnosing hepatic fibrosis,7–9 few have explored the impact of different numbers of cardiometabolic risk factors (CMRFs) on diagnostic accuracy among patients with CHB and MASLD. Therefore, we aimed to evaluate the accuracy of these NITs in identifying significant hepatic fibrosis in this specific population.
This multi-center, retrospective study was conducted at eleven Chinese hospitals (ClinicalTrials.gov identifier: NCT05766449). CHB was defined as hepatitis B surface antigen positive for ≥6 months. HS was diagnosed as steatosis exceeding 5% confirmed by liver biopsy. In this study, MASLD was defined as biopsy-proven HS in combination with one or more of the following CMRFs: (1) BMI ≥ 25 kg/m2; (2) fasting plasma glucose ≥5.6 mmol/L or diagnosed type 2 diabetes mellitus (T2DM); (3) hypertension; (4) plasma triglyceride ≥1.70 mmol/L; (5) plasma high-density lipoprotein cholesterol ≤1.0/1.3 mmol/L for males/females. The exclusion criteria included: (1) excessive alcohol intake (ongoing or recent alcohol consumption over 30/20 g of alcohol per day for men/women)10; (2) co-infection with other viruses; (3) presence of other liver diseases; (4) diagnosis of malignancies; (5) insufficient clinical data. Liver fibrosis was categorized into S0-S4 according to Scheuer’s classification, with ≥S2 defined as significant fibrosis.11 To ensure data relevancy and consistency, only laboratory examinations conducted within a 14-day window before the liver biopsy were considered. The population was classified into three groups: group A of CHB patients with simple HS, group B of CHB patients with MASLD involving 1–3 CMRFs, and group C of CHB patients with MASLD involving 4–5 CMRFs. The different optimal cut-off values of FIB-4, NFS, and APRI in the three groups were determined by the Youden index. Differences in the area under the curve (AUC) were compared using the DeLong test.
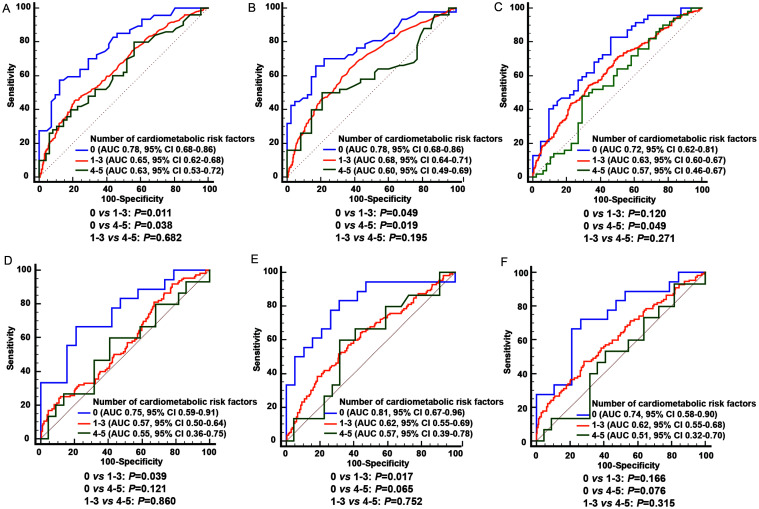
A total of 1,063 eligible patients were enrolled, with 47 patients (53.4%), 406 patients (46.3%), and 50 patients (51.0%) in groups A, B, and C diagnosed as ≥S2 confirmed by liver biopsy, respectively. The population characteristics of the whole cohort are available in Supplementary Table 1. The optimal cutoff values of the three NITs, along with the corresponding sensitivity, specificity, positive predictive value, and negative predictive value in the three groups, are presented in Supplementary Table 2. For diagnostic performance, the AUCs of FIB-4, APRI, and NFS in diagnosing ≥S2 within group A were 0.78, 0.78, and 0.72, respectively (Fig. 1). However, in CHB patients with MASLD, the accuracy of the three NITs decreased with an increasing number of CMRFs. The AUCs of FIB-4, APRI, and NFS were 0.65, 0.68, and 0.63 in group B, respectively. These AUCs further declined in group C, all falling below 0.63. FIB-4 and APRI both demonstrated significantly poorer diagnostic performance for group B and group C compared to group A (all p < 0.05, Fig. 1), and the AUC of NFS was significantly lower in group C compared with group A (p < 0.05, Fig. 1). In the subgroup with normal ALT levels (<40 IU/L), a consistent trend was observed. As the number of CMRFs increased, the AUC of the three NITs decreased. Significant differences were achieved when comparing the AUCs of FIB-4 and APRI between groups A and B (both p < 0.05, Fig. 1).
Fig. 1. The diagnostic performance of the three non-invasive tests for significant fibrosis (≥S2) in CHB patients with HS or MASLD.
(A) The AUC of FIB-4 for all patients, (B) The AUC of APRI for all patients, (C) The AUC of NFS for all patients, (D) The AUC of FIB-4 in patients with normal ALT levels, (E) The AUC of APRI in patients with normal ALT levels, (F) The AUC of NFS in patients with normal ALT levels. Normal ALT levels are defined as ALT < 40 IU/L. HS, hepatic steatosis; MASLD, metabolic dysfunction-associated steatotic liver disease; FIB-4, the fibrosis-4 index; APRI, the aspartate aminotransferase-to-platelet ratio index; NFS, the non-alcoholic fatty liver disease fibrosis score; AUC, area under the curve; CHB, chronic hepatitis B; ALT, alanine aminotransferase.
In this study, we found that the diagnostic accuracy of the three NITs diminished as the number of CMRFs increased in CHB patients with MASLD. NITs have emerged as valuable tools for diagnosing and prognosing chronic liver disease. However, the diagnostic accuracy can vary when patients have different CMRFs. A retrospective study by Boursier J et al.12 reported that the AUCs of FIB-4 and NFS for identifying advanced fibrosis were significantly lower in NAFLD patients with T2DM compared to those without T2DM. Furthermore, an individual patient data meta-analysis involving 5,735 NAFLD patients confirmed that FIB-4 and NFS performed better in patients with lower BMI and without T2DM.13 These findings align with our results, suggesting that CMRFs potentially impact the diagnostic performance of NITs. The three existing NITs mainly utilize transaminase levels, platelet counts, and other available variables that indirectly reflect liver injury. Since MASLD often lacks specific symptoms for a long period, scores calculated from these NITs with limited specificity may mask the progression of liver disease. In the context of CHB combined with MASLD, metabolic dysregulation could exacerbate liver fibrosis. Hepatocyte damage may result in the release of inflammatory cytokines, triggering hepatic stellate cell activation and proliferation.14 Excessive fat accumulation and oxidative stress can promote pro-fibrotic and carcinogenic processes.14 Abnormal lipid metabolism induced by HBsAg-related promyelocytic leukemia protein deficiency may accelerate the progression of liver-related adverse events.14 The complex interplay among CHB, MASLD, and other CMRFs necessitates further investigation.
Currently, some biomarkers or panels have been developed to directly reflect the severity of liver fibrosis by measuring circulating markers released during fibrogenesis or extracellular matrix remodeling, such as N-terminal propeptide of type 3 collagen, enhanced liver fibrosis, and FibroTest.15 These novel tests show promise for more precise diagnosis in populations with chronic liver disease.
This study has some limitations. Firstly, as a retrospective study, the data were collected before the nomenclature of MASLD was proposed. Therefore, some indicators used to evaluate CMRFs are missing, such as waist circumference and two-hour postprandial blood glucose. Secondly, the sample sizes of group A (0 CMRF) and group C (4–5 CMRFs) are too small, limiting the potential for more detailed analysis. Thirdly, pathological signs of steatohepatitis, such as hepatic ballooning or Mallory bodies, were not strictly recorded in patients.
In conclusion, this study showed a notable decrease in the diagnostic accuracy of three NITs for significant fibrosis in patients with concomitant MASLD and CHB, especially in those with an elevated number of CMRFs. Future research is needed to improve the diagnostic performance of NITs in this population. Developing personalized algorithms for specific patients and exploring the impact of various metabolic factors on NITs are necessary to optimize liver fibrosis risk stratification in CHB patients with MASLD.
Supporting information
Acknowledgments
We thank Qi Zheng, Qinglei Zeng, Zebao He, Yuanwang Qiu, Chuanwu Zhu, and Weimao Ding for data support.
Ethical statement
This multi-center, retrospective study was conducted at eleven Chinese hospitals (ClinicalTrials.gov identifier: NCT05766449). We have received approval from the Institutional Ethics Review Board of all the involved hospitals, with document numbers of 2008022. This study was carried out in accordance with the Declaration of Helsinki. The informed consent was waived for a retrospective study.
Data sharing statement
We are unable to provide access to our data for privacy reasons. The protocol and statistical analysis methods used in the study can be requested directly from the corresponding author after approval.
References
- 1.Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–398. doi: 10.1016/S2468-1253(19)30039-1,. [DOI] [PubMed] [Google Scholar]
- 2.Zhou R, Yang L, Zhang B, Gu Y, Kong T, Zhang W, et al. Clinical impact of hepatic steatosis on chronic hepatitis B patients in Asia: A systematic review and meta-analysis. J Viral Hepat. 2023;30(10):793–802. doi: 10.1111/jvh.13872. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Q, Zou B, Wu Y, Yeo Y, Wu H, Stave CD, et al. Systematic review with meta-analysis: prevalence of hepatic steatosis, fibrosis and associated factors in chronic hepatitis B. Aliment Pharmacol Ther. 2021;54(9):1100–1109. doi: 10.1111/apt.16595. [DOI] [PubMed] [Google Scholar]
- 4.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–36. doi: 10.1002/hep.21669,. [DOI] [PubMed] [Google Scholar]
- 5.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 6.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–854. doi: 10.1002/hep.21496,. [DOI] [PubMed] [Google Scholar]
- 7.Castellana M, Donghia R, Guerra V, Procino F, Castellana F, Zupo R, et al. Fibrosis-4 Index vs Nonalcoholic Fatty Liver Disease Fibrosis Score in Identifying Advanced Fibrosis in Subjects With Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Am J Gastroenterol. 2021;116(9):1833–1841. doi: 10.14309/ajg.0000000000001337. [DOI] [PubMed] [Google Scholar]
- 8.López Tórrez SM, Ayala CO, Ruggiro PB, Costa CAD, Wagner MB, Padoin AV, et al. Accuracy of prognostic serological biomarkers in predicting liver fibrosis severity in people with metabolic dysfunction-associated steatotic liver disease: a meta-analysis of over 40,000 participants. Front Nutr. 2024;11:1284509. doi: 10.3389/fnut.2024.1284509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vali Y, Lee J, Boursier J, Petta S, Wonders K, Tiniakos D, et al. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): a comparative diagnostic accuracy study. Lancet Gastroenterol Hepatol. 2023;8(8):714–725. doi: 10.1016/S2468-1253(23)00017-1. [DOI] [PubMed] [Google Scholar]
- 10.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 11.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13(3):372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 12.Boursier J, Canivet CM, Costentin C, Lannes A, Delamarre A, Sturm N, et al. Impact of Type 2 Diabetes on the Accuracy of Noninvasive Tests of Liver Fibrosis With Resulting Clinical Implications. Clin Gastroenterol Hepatol. 2023;21(5):1243–1251.e12. doi: 10.1016/j.cgh.2022.02.059. [DOI] [PubMed] [Google Scholar]
- 13.Mózes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. 2022;71(5):1006–1019. doi: 10.1136/gutjnl-2021-324243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong X, Song Y, Yin S, Wang J, Huang R, Wu C, et al. Clinical impact and mechanisms of hepatitis B virus infection concurrent with non-alcoholic fatty liver disease. Chin Med J (Engl) 2022;135(14):1653–1663. doi: 10.1097/CM9.0000000000002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anstee QM, Castera L, Loomba R. Impact of non-invasive biomarkers on hepatology practice: Past, present and future. J Hepatol. 2022;76(6):1362–1378. doi: 10.1016/j.jhep.2022.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.