Table 1. Biological Activities of the Synthesized 5-Aminothiazolesi.
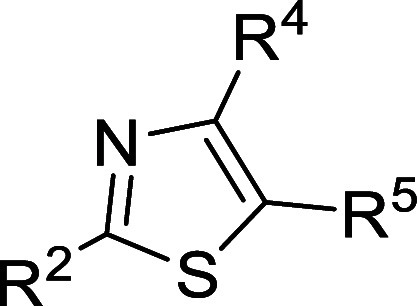
| compound | R2 | R4 | R5 | IC50 (nM)a (95% CI) | αSyn dimerization at 10 μM (%)b | autophagy at 10 μM (%)c | ROS at 10 μM (%)d | |
|---|---|---|---|---|---|---|---|---|
| KYP-2047e | <1f | 87 ± 1 | 89 ± 3 | 88 ± 3 | ||||
| HUP-28g | 130 | (71–230) | 75 ± 5 | 71 ± 6 | 95 ± 7 | |||
| HUP-55e | 5.0 | (3.2–7.6) | 85 ± 2 | 87 ± 3 | 85 ± 3 | |||
| HUP-46 | Ph(CH2)3– | Me | (S)-2-cyanopyrrolidin-1-yl | 8000 | (4900–13,000) | 74 ± 2 | 77 ± 3 | 85 ± 11 |
| 7 | Ph(CH2)3– | Me | pyrrolidin-1-yl | 4800 | (2100–11,000) | 74 ± 2 | 87 ± 4 | 75 ± 9 |
| 8 | Ph(CH2)3– | Me | piperidin-1-yl | 210,000 | (74,000–590,000) | 82 ± 11 | 106 ± 10 | 73 ± 26 |
| 9 | Ph(CH2)3– | Me | (R)-3-fluoropyrrolidin-1-yl | 97,000 | (25,000–380,000) | 95 ± 1 | 95 ± 2 | n.d. |
| 10 | Ph(CH2)3– | Me | (S)-2-hydroxymethylpyrrolidin-1-yl | 95,000 | (35,000–260,000) | 99 ± 19 | 96 ± 3 | n.d. |
| 11h | Ph(CH2)3– | Me | (S)-2-carbamoylpyrrolidin-1-yl | 80,000 | (15,000–420,000) | 94 ± 8 | 84 ± 3 | 86 ± 4 |
| 12 | Ph(CH2)2– | Me | pyrrolidin-1-yl | 47,000 | (18,000–120,000) | 107 ± 15 | 89 ± 2 | 59 ± 18 |
| 13 | Ph(CH2)3– | Me | 2-tetrazolylpyrrolidin-1-yl | n.d. | n.d. | 111 ± 10 | 98 ± 5 | 86 ± 11 |
| 16a | 3,4-dimethoxyphenyl–(CH2)3– | Me | pyrrolidin-1-yl | 12,000 | (4100–33,000) | 92 ± 5 | 83 ± 6 | 48 ± 3 |
| 16b | 4-cyanophenyl–(CH2)3– | Me | pyrrolidin-1-yl | 340 | (68–1700) | 113 ± 9 | 96 ± 1 | 53 ± 4 |
| 16c | 4-iodophenyl–(CH2)3– | Me | pyrrolidin-1-yl | 9800 | (2600–38,000) | n.d. | 97 ± 3 | n.d. |
| 16d | 2-pyridyl–(CH2)3– | Me | pyrrolidin-1-yl | 7300 | (4800–11,000) | 96 ± 7 | 104 ± 4 | 57 ± 2 |
| 16e | 3-pyridyl–(CH2)3– | Me | pyrrolidin-1-yl | 7900 | (5700–11,000) | 105 ± 3 | 102 ± 5 | 60 ± 3 |
| 16f | 3-pyridyl–(CH2)2– | Me | pyrrolidin-1-yl | 3300 | (2000–5300) | 96 ± 8 | 108 ± 3 | 62 ± 5 |
| 16g | 3-indolyl–(CH2)2– | Me | pyrrolidin-1-yl | 5700 | (3700–8600) | 105 ± 3 | 83 ± 5 | 55 ± 3 |
| 16h | 1-benzimidazolyl–(CH2)2– | Me | pyrrolidin-1-yl | 62,400 | (37,680–103,200) | 86 ± 8 | 78 ± 5 | n.d. |
| 16i | 2-benzimidazolyl–(CH2)2– | Me | pyrrolidin-1-yl | 2100 | (730–6300) | 93 ± 2 | 101 ± 6 | 59 ± 3 |
| 16j | Ph(CH2)3– | isopropyl | pyrrolidin-1-yl | 4000 | (2600–6100) | 90 ± 6 | 97 ± 3 | 85 ± 5 |
| 16k | 3-indolyl–(CH2)2– | isopropyl | pyrrolidin-1-yl | 5800 | (12,000–79,000) | 105 ± 8 | 100 ± 6 | 83 ± 4 |
| 16l | Ph(CH2)3– | H | pyrrolidin-1-yl | 126,000 | (49,350–340,500) | 125 ± 21 | 87 ± 4 | n.d. |
| 17 | N-methyl-3-indolyl–(CH2)2– | Me | pyrrolidin-1-yl | 3900 | (1900–7700) | 89 ± 8 | 103 ± 3 | n.d. |
Assessed using recombinant porcine PREP with Suc-Gly-Pro-AMC as the substrate.
Luminescence signal percentage of DMSO control with SEM, assessed with a split Gaussia luciferase-based method using Neuro2A cells.
GFP signal percentage of DMSO control with SEM, assessed using HEK-293 cells stably expressing GFP-LC3B.
Fluorescence signal percentage of DMSO control with SEM, assessed using a fluorogenic ROS assay.
Results reported by Kilpeläinen et al.18
The assay is limited by the enzyme concentration of 2 nM for IC50 values under this concentration; KYP-2047 is a slow, tight-binding inhibitor with a Ki-value of 0.02 nM.25,26
Synthesis intermediate.
n.d.: not determined.
